A July 12, 2016 news item on phys.org describes a project that could lead to the first carbon nanotube mirrors to be used in a Cubesat telescope in space,
A lightweight telescope that a team of NASA scientists and engineers is developing specifically for CubeSat scientific investigations could become the first to carry a mirror made of carbon nanotubes in an epoxy resin.
Led by Theodor Kostiuk, a scientist at NASA’s [US National Aeronautics and Space Administration] Goddard Space Flight Center in Greenbelt, Maryland, the technology-development effort is aimed at giving the scientific community a compact, reproducible, and relatively inexpensive telescope that would fit easily inside a CubeSat. Individual CubeSats measure four inches on a side.
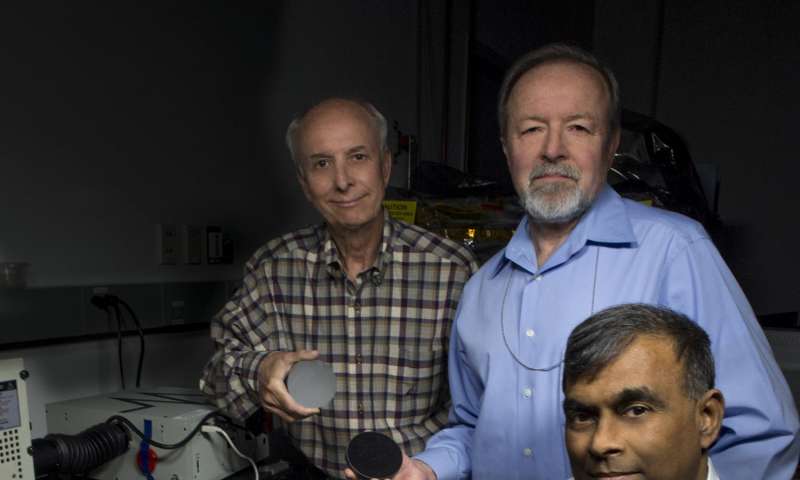
John Kolasinski (left), Ted Kostiuk (center), and Tilak Hewagama (right) hold mirrors made of carbon nanotubes in an epoxy resin. The mirror is being tested for potential use in a lightweight telescope specifically for CubeSat scientific investigations. Credit: NASA/W. Hrybyk
A July 12, 2016 US National Aeronautics and Space Administration (NASA) news release, which originated the news item, provides more information about Cubesats,
Small satellites, including CubeSats, are playing an increasingly larger role in exploration, technology demonstration, scientific research and educational investigations at NASA. These miniature satellites provide a low-cost platform for NASA missions, including planetary space exploration; Earth observations; fundamental Earth and space science; and developing precursor science instruments like cutting-edge laser communications, satellite-to-satellite communications and autonomous movement capabilities. They also allow an inexpensive means to engage students in all phases of satellite development, operation and exploitation through real-world, hands-on research and development experience on NASA-funded rideshare launch opportunities.
Under this particular R&D effort, Kostiuk’s team seeks to develop a CubeSat telescope that would be sensitive to the ultraviolet, visible, and infrared wavelength bands. It would be equipped with commercial-off-the-shelf spectrometers and imagers and would be ideal as an “exploratory tool for quick looks that could lead to larger missions,” Kostiuk explained. “We’re trying to exploit commercially available components.”
While the concept won’t get the same scientific return as say a flagship-style mission or a large, ground-based telescope, it could enable first order of scientific investigations or be flown as a constellation of similarly equipped CubeSats, added Kostiuk.
With funding from Goddard’s Internal Research and Development program, the team has created a laboratory optical bench made up of three commercially available, miniaturized spectrometers optimized for the ultraviolet, visible, and near-infrared wavelength bands. The spectrometers are connected via fiber optic cables to the focused beam of a three-inch diameter carbon-nanotube mirror. The team is using the optical bench to test the telescope’s overall design.
The news release then describes the carbon nanotube mirrors,
By all accounts, the new-fangled mirror could prove central to creating a low-cost space telescope for a range of CubeSat scientific investigations.
Unlike most telescope mirrors made of glass or aluminum, this particular optic is made of carbon nanotubes embedded in an epoxy resin. Sub-micron-size, cylindrically shaped, carbon nanotubes exhibit extraordinary strength and unique electrical properties, and are efficient conductors of heat. Owing to these unusual properties, the material is valuable to nanotechnology, electronics, optics, and other fields of materials science, and, as a consequence, are being used as additives in various structural materials.
“No one has been able to make a mirror using a carbon-nanotube resin,” said Peter Chen, a Goddard contractor and president of Lightweight Telescopes, Inc., a Columbia, Maryland-based company working with the team to create the CubeSat-compatible telescope.
“This is a unique technology currently available only at Goddard,” he continued. “The technology is too new to fly in space, and first must go through the various levels of technological advancement. But this is what my Goddard colleagues (Kostiuk, Tilak Hewagama, and John Kolasinski) are trying to accomplish through the CubeSat program.”
…
The use of a carbon-nanotube optic in a CubeSat telescope offers a number of advantages, said Hewagama, who contacted Chen upon learning of a NASA Small Business Innovative Research program awarded to Chen’s company to further advance the mirror technology. In addition to being lightweight, highly stable, and easily reproducible, carbon-nanotube mirrors do not require polishing — a time-consuming and often times expensive process typically required to assure a smooth, perfectly shaped mirror, said Kolasinski, an engineer and science collaborator on the project.
To make a mirror, technicians simply pour the mixture of epoxy and carbon nanotubes into a mandrel or mold fashioned to meet a particular optical prescription. They then heat the mold to to cure and harden the epoxy. Once set, the mirror then is coated with a reflective material of aluminum and silicon dioxide.
“After making a specific mandrel or mold, many tens of identical low-mass, highly uniform replicas can be produced at low cost,” Chen said. “Complete telescope assemblies can be made this way, which is the team’s main interest. For the CubeSat program, this capability will enable many spacecraft to be equipped with identical optics and different detectors for a variety of experiments. They also can be flown in swarms and constellations.”
There could be other applications for these carbon nanotube mirrors according to the news release,
A CubeSat telescope is one possible application for the optics technology, Chen added.
He believes it also would work for larger telescopes, particularly those comprised of multiple mirror segments. Eighteen hexagonal-shape mirrors, for example, form the James Webb Space Telescope’s 21-foot primary mirror and each of the twin telescopes at the Keck Observatory in Mauna Kea, Hawaii, contain 36 segments to form a 32-foot mirror.
Many of the mirror segments in these telescopes are identical and can therefore be produced using a single mandrel. This approach avoids the need to grind and polish many individual segments to the same shape and focal length, thus potentially leading to significant savings in schedule and cost.
Moreover, carbon-nanotube mirrors can be made into ‘smart optics’. To maintain a single perfect focus in the Keck telescopes, for example, each mirror segment has several externally mounted actuators that deform the mirrors into the specific shapes required at different telescope orientations.
In the case of carbon-nanotube mirrors, the actuators can be formed into the optics at the time of fabrication. This is accomplished by applying electric fields to the resin mixture before cure, which leads to the formation of carbon-nanotube chains and networks. After curing, technicians then apply power to the mirror, thereby changing the shape of the optical surface. This concept has already been proven in the laboratory.
“This technology can potentially enable very large-area technically active optics in space,” Chen said. “Applications address everything from astronomy and Earth observing to deep-space communications.”
Dexter Johnson provides some additional tidbits in his July 14, 2016 post (on his Nanoclast blog on the IEEE [Institute for Electrical and Electronics Engineers] about the Cubesat mirrors.