It’s been a while since I’ve seen Pulickel Ajayan’s name in a Rice University (Texas) news release and I wonder if this is the beginning of a series. I’ve noticed that researchers often publish a series of papers within a few months and then become quiet for two or more years as they work in their labs to gather more information.
This time the research from Pulickel’s lab has focused on the use of carbon nanotubes to harvest water from desert air. From a June 12, 2014 news item on Azonano,
If you don’t want to die of thirst in the desert, be like the beetle. Or have a nanotube cup handy.
New research by scientists at Rice University demonstrated that forests of carbon nanotubes can be made to harvest water molecules from arid desert air and store them for future use.
The invention they call a “hygroscopic scaffold” is detailed in a new paper in the American Chemical Society journal Applied Materials and Interfaces.
Researchers in the lab of Rice materials scientist Pulickel Ajayan found a way to mimic the Stenocara beetle, which survives in the desert by stretching its wings to capture and drink water molecules from the early morning fog.
Here’s more about the research from a June 11, 2014 Rice University news release (by Mike Williams?), which originated the news item,
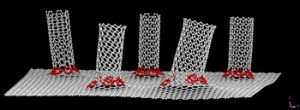
They modified carbon nanotube forests grown through a process created at Rice, giving the nanotubes a superhydrophobic (water-repelling) bottom and a hydrophilic (water loving) top. The forest attracts water molecules from the air and, because the sides are naturally hydrophobic, traps them inside.
“It doesn’t require any external energy, and it keeps water inside the forest,” said graduate student and first author Sehmus Ozden. “You can squeeze the forest to take the water out and use the material again.”
The forests grown via water-assisted chemical vapor deposition consist of nanotubes that measure only a few nanometers (billionths of a meter) across and about a centimeter long.
The Rice team led by Ozden deposited a superhydrophobic layer to the top of the forest and then removed the forest from its silicon base, flipped it and added a layer of hydrophilic polymer to the other side.
In tests, water molecules bonded to the hydrophilic top and penetrated the forest through capillary action and gravity. (Air inside the forest is compressed rather then expelled, the researchers assumed.) Once a little water bonds to the forest canopy, the effect multiplies as the molecules are drawn inside, spreading out over the nanotubes through van der Waals forces, hydrogen bonding and dipole interactions. The molecules then draw more water in.
The researchers tested several variants of their cup. With only the top hydrophilic layer, the forests fell apart when exposed to humid air because the untreated bottom lacked the polymer links that held the top together. With a hydrophilic top and bottom, the forest held together but water ran right through.
But with a hydrophobic bottom and hydrophilic top, the forest remained intact even after collecting 80 percent of its weight in water.
The amount of water vapor captured depends on the air’s humidity. An 8 milligram sample (with a 0.25-square-centimeter surface) pulled in 27.4 percent of its weight over 11 hours in dry air, and 80 percent over 13 hours in humid air. Further tests showed the forests significantly slowed evaporation of the trapped water.
If it becomes possible to grow nanotube forests on a large scale, the invention could become an efficient, effective water-collection device because it does not require an external energy source, the researchers said.
Ozden said the production of carbon nanotube arrays at a scale necessary to put the invention to practical use remains a bottleneck. “If it becomes possible to make large-scale nanotube forests, it will be a very easy material to make,” he said.
This is not the first time researchers have used the Stenocara beetle (also known as the Namib desert beetle) as inspiration for a water-harvesting material. In a Nov. 26, 2012 posting I traced the inspiration back to 2001 while featuring the announcement of a new startup company,
… US startup company, NBD Nano, which aims to bring a self-filling water bottle based on Namib desert beetle to market,
NBD Nano, which consists of four recent university graduates and was formed in May [2012], looked at the Namib Desert beetle that lives in a region that gets about half an inch of rainfall per year.
Using a similar approach, the firm wants to cover the surface of a bottle with hydrophilic (water-attracting) and hydrophobic (water-repellent) materials.
The work is still in its early stages, but it is the latest example of researchers looking at nature to find inspiration for sustainable technology.
“It was important to apply [biomimicry] to our design and we have developed a proof of concept and [are] currently creating our first fully-functional prototype,” Miguel Galvez, a co-founder, told the BBC.
“We think our initial prototype will collect anywhere from half a litre of water to three litres per hour, depending on local environments.”
You can find out more about NBD Nano here although they don’t give many details about the material they’ve developed. Given that MIT (Massachusetts Institute of Technology) researchers published a paper about a polymer-based material laced with silicon nanoparticles inspired by the Namib beetle in 2006 and that NBD Nano is based Massachusetts, I believe NBD Nano is attempting to commercialize the material or some variant developed at MIT.
Getting back to Rice University and carbon nanotubes, this is a different material attempting to achieve the same goal, harvesting water from desert air. Here’s a link to and a citation for the latest paper inspired by the Stenocara beetle (Namib beetle),
Anisotropically Functionalized Carbon Nanotube Array Based Hygroscopic Scaffolds by Sehmus Ozden, Liehui Ge , Tharangattu N. Narayanan , Amelia H. C. Hart , Hyunseung Yang , Srividya Sridhar , Robert Vajtai , and Pulickel M Ajayan. ACS Appl. Mater. Interfaces, DOI: 10.1021/am5022717 Publication Date (Web): June 4, 2014
Copyright © 2014 American Chemical Society
This paper is behind a paywall.
One final note, the research at MIT was funded by DARPA (US Defense Advanced Research Projects Agency). According to the news release the Rice University research held interest for similar agencies,
The U.S. Department of Defense and the U.S. Air Force Office of Scientific Research Multidisciplinary University Research Initiative supported the research.
![NanoOrchard – Electrochemically overgrown CuNi nanopillars. (Image courtesy of the Materials Research Society Science as Art Competition and Josep Nogues, Institut Catala de Nanociencia i Nanotecnologia (ICN2), Spain, and A. Varea, E. Pellicer, S. Suriñach, M.D. Baro, J. Sort, Univ. Autonoma de Barcelona) [downloaded from http://www.nanowerk.com/nanotechnology-news/newsid=35631.php]](http://www.frogheart.ca/wp-content/uploads/2014/05/NanoOrchard-300x198.jpg)
![Armchair carbon nanotubes, so named for the arrangement of atoms that make their ends look like armchairs, are the most desirable among nanotube researchers for their superior electrical properties. Image by Erik Hároz [downloaded from http://news.rice.edu/2013/02/05/essential-armchair-reading-for-nanotube-researchers-2/]](http://www.frogheart.ca/wp-content/uploads/2013/02/ArmchairCarbonNanotube-300x251.jpeg)