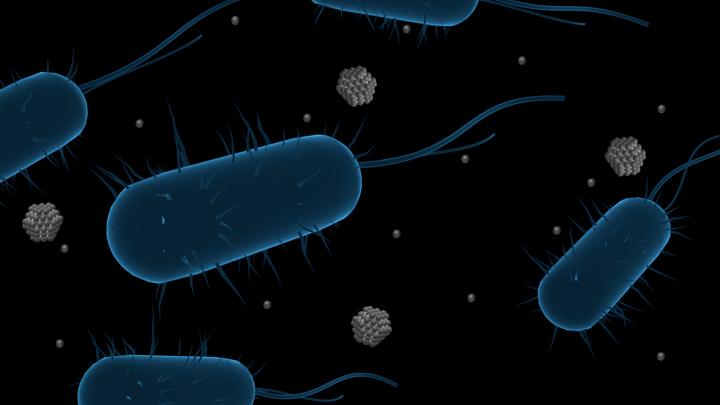
This work is not about drinking tea with silver nanoparticles in it or ingesting colloidal silver by any means, a dangerous practice as Nicole Karlis’s January 7, 2024 article for Salon highlights, Note: Links have been removed,
The HBO docuseries “Love Has Won: The Cult of Mother God” begins with a jarring image. The corpse of the cult leader, Amy Carlson, laying in a bed, wrapped in blankets and string lights. She is noticeably gaunt and her face is a very blue color. When Carlson died in 2021 at the age of 45, a coroner’s report deemed her cause of death to be “alcohol abuse, anorexia and chronic colloidal silver ingestion.”
…
Most medical experts advise against ingesting silver — especially in large amounts. That’s because too much of it can build up in a person’s body and lead to argyria, which is the condition that Carlson and Stan Jones both had that turned them a blue. While argyria alone isn’t a serious health condition, it doesn’t go away when a person stops ingesting silver. Plus, too much silver can be fatal. [emphasis mine]
…
A November 17, 2023 news item on phys.org announced research from the Polish Academy of Sciences into improving antimicrobial activity, Note: A link has been removed,
Researchers at the Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) have demonstrated that green tea–silver nanoparticles as a powerful tool against pathogens such as bacteria and yeast. Their work is published in Nanoscale Advances.
…
An undated Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) press release (also published on EurekAlert and dated November 17, 2023), which originated the news item, describes this work, which is intended for medical applications, in more detail,
Once upon a time, people believed to be invincible against bacterial diseases, thanks to the antibiotics. Does this sound like a fairy tale? By all means! Nothing could be further from the truth. Despite widespread access to antibiotic therapy, many lives are lost due to pathogens invisible to the eye. The ability to develop drugs that can combat resistant strains of bacteria has not kept pace with the spread of resistance. So far, innovations to defeat antimicrobial-resistant strains of bacteria are in high demand. Recently, researchers at the Institute of Physical Chemistry of the Polish Academy of Sciences (IPC PAS) demonstrated green tea-silver nanoparticles as a powerful tool against pathogens such as bacteria and yeast. Their goal was to develop an efficient method to combat bacteria that are otherwise unaffected by antimicrobial agents, such as antibiotics.
Following the discovery of antibiotics, there came a change in the curse of mankind by accelerating the development of medicine and extending human life expectancy. Their successful implementation led to the rapid development of pharmacy, providing more and more drugs against many pathogens. Nevertheless, the overuse of antibiotics has led to the emergence of resistance to these compounds, becoming one of the biggest health threats worldwide. As a result, antibiotic resistance has emerged faster than the advancement of antibiotics . The appearance of new drugs on the horizon to combat these pathogens is a short-lasting spark. Even if we seem to be on the losing end, there is still a chance to defeat an invisible enemy.
This hitch was researched by the team of scientists from the IPC PAS under the supervision of Prof. Jan Paczesny, who proposed new nanoformulations for use against widespread and challenging pathogens such as ESKAPE bacteria (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.) and other problematic yeast pathogens such as Candida auris or Cryptococcus neoformans. These microorganisms, treated with commercially available antibiotics, rapidly develop antibiotic resistance. Researchers chose ESKAPE as the target group since these pathogens lead to serious diseases, from sepsis to even cancer. How? This is where the story begins.
A few months ago, Paczesny’s team decided to try combining silver nanoparticles, which are known for their antimicrobial and antifungal properties, and tea extracts rich in polyphenols additionally possessing antioxidant properties. The concept was built to enhance broad-spectrum efficacy against pathogens using green hybrid silver nanoparticles (AgNPs), which are significantly more effective than all ingredients and even more effective than certain antibiotics. Why are these hybrid particles so special? In their work, three well-known tea varieties: black tea (B-Tea), green tea (G-Tea) and Pu-erh tea (R-Tea) were used as a capping agent, which acts as a stabilizer to protect the synthesized particles from aggregation. In this way, the particles offer a high active surface area compared to other formulations. Additionally, such synthesis is eco-friendly for the use of natural ingredients during precipitation. The structures produced vary in shape and size from 34 to 65 nm, depending on the type of tea used during synthesis, and show different reactivity towards microorganisms.
Initially, silver nanoparticles produced in the presence of tea extracts (B-TeaNPs, G-TeaNPs and R-TeaNPs) were used to treat Gram-negative (E. coli) and Gram-positive (E. faecium) bacterial strains to test the effect on strains with different cell envelope morphologies. They looked at the interactions between the manufactured nanoparticles and the pathogens to determine efficacy, comparing the results with commercially available antibiotics. The ESKAPE pathogens were then tested according to a protocol for the most effective concentration and composition of the particles, revealing up to a 25% decrease in the number of bacterial cells in E. faecium and a 90% decrease in the case of E. cloacae. Interestingly, the green silver nanoparticles also showed antifungal activity, leading to an 80% decrease in the number of viable cells of C. auris and about a 90% decrease for C. neoformans.
The first author, Sada Raza claims “What is more, the size of nanoparticles is usually related to the cytotoxic effect of nanomaterials, with smaller particles being more cytotoxic. This should favor control AgNPs and R-TeaNPs over G-TeaNPs and B-TeaNPs in our experiments. This was not the case. In most experiments, C-AgNPs and R-TeaNPs showed the lowest antimicrobial efficacy. This is in line with other studies, which demonstrated that size is not a primary factor affecting the antimicrobial activity of AgNPs.”
The antibacterial and antifungal properties of silver nanoparticles made with tea extracts are greater than those of silver nanoparticles alone due to their high content of phenolic compounds, isoflavonoids (especially catechins such as epigallocatechin (EGC) and epigallocatechin gallate (EGCG)). These combinations, using biologically active tea extracts and smaller amounts of silver nanoparticles, appear to be a potential way to combat a range of infections and even replace antibiotics in some applications.
“We established that silver nanoparticles synthesized with tea extracts have higher antibacterial properties than silver nanoparticles alone. Therefore, lower dosages of TeaNPs could be used (0.1 mg mL−1). We confirmed that in some cases, the synergistic effect of tea extracts and silver nanoparticles allowed for efficacy higher than that of antibiotics (ampicillin) when tested at the same concentrations (0.1 mg mL−1) and after a relatively short exposure time of three hours.” – remarks Mateusz Wdowiak, co-author of this work.
The researchers found that the antimicrobial hybrid nanoparticles resulted in a significant reduction in bacteria compared to antibiotics or compounds separately. Although not all bacteria were killed, this is a significant improvement that could aid the treatment of superbugs using much lower doses than other commercially available compounds. The amount of hybrid silver nanoparticles needed to overcome bacteria or fungal infections is extremely low, making them cost-effective, so the key to using them well is not only functionality, but also the low cost of application.
It is an approach that can also be adapted to combat other difficult-to-treat bacterial infections. The new nanoparticles developed by researchers at the IPC PAS could bring us one step closer to effectively killing deadly drug-resistant superbugs, providing an alternative to antibiotics against Gram-negative and Gram-positive bacteria. This study also shows how much more work there is to be done in this field. Compounds used separately were much less effective than the green hybrid.
In the future, the researchers’ main goal is to use nanoparticles in everyday life, starting with agricultural applications, replacing harmful compounds used in fields to overcome infestations in plants and bring us closer to organic farming. On a larger scale, the proposed material could also be used in biomedical applications, such as an additive for wound dressings to protect against Gram-negative and Gram-positive bacteria. They hope to use nanotechnology to develop more targeted treatments for drug-resistant superbugs.
Their work was published in Nanoscale Advances journal and was financed by the National Science Centre, Poland, within the SONATA BIS grant number 2017/26/E/ST4/00041 and Foundation for Polish Science from the European Regional Development Fund within the project POIR.04.04.00-00-14D6/18-00 ‘Hybrid sensor platforms for integrated photonic systems based on ceramic and polymer materials (HYPHa)’ (TEAM-NET program).
Here’s a link to and a citation for the paper,
Enhancing the antimicrobial activity of silver nanoparticles against ESKAPE bacteria and emerging fungal pathogens by using tea extracts by Sada Raza, Mateusz Wdowiak, Mateusz Grotek, Witold Adamkiewicz, Kostiantyn Nikiforow, Pumza Mente, and Jan Paczesny. Nanoscale Adv., 2023,5, 5786-5798 DOI: https://doi.org/10.1039/D3NA00220A
This paper is licensed under a Creative Commons Attribution 3.0 Unported Licence. “You can use material from this article in other publications without requesting further permissions from the RSC [Royal Society of Chemistry], provided that the correct acknowledgement is given.” Or, consider it an open access paper.
Finally, this is not a recommendation not is it an endorsement for the ingestion of colloidal silver.