I like pictures of happy researchers and, as these pictures go, the researchers seem pretty relaxed,

A March 23, 2023 Virginia Commonwealth University (VCU) news release (also on EurekAlert) announces work into making corneal transplants more successful, Note: A link has been removed,
Corneal transplants can be the last step to returning clear vision to many patients suffering from eye disease. Each year, approximately 80,000 corneal transplantations take place in the U.S. Worldwide, more than 184,000 corneal transplantation surgeries are performed annually.
However, rejection rates for the corneal grafts can be as high as 10%. This is largely due to poor patient compliance to the medications, which require frequent administrations of topical eyedrops over a long period of time.
This becomes especially acute when patients show signs of early rejection of the transplanted corneas. When this occurs, patients need to apply topical eyedrops [sic] hourly to rescue the corneal grafts from failure.
The tedious process of eyedrop [sic] dosing causes a tremendous burden for patients. The resulting noncompliance to medication treatment can lead to even higher graft-rejection rates.
Research led by a team at Virginia Commonwealth University may make the corneal grafts more successful by using nanoparticles to encapsulate the medication. The novel approach could significantly improve patient compliance, according to a paper recently published in Science Advances, “Six-month effective treatment of corneal graft rejection.”
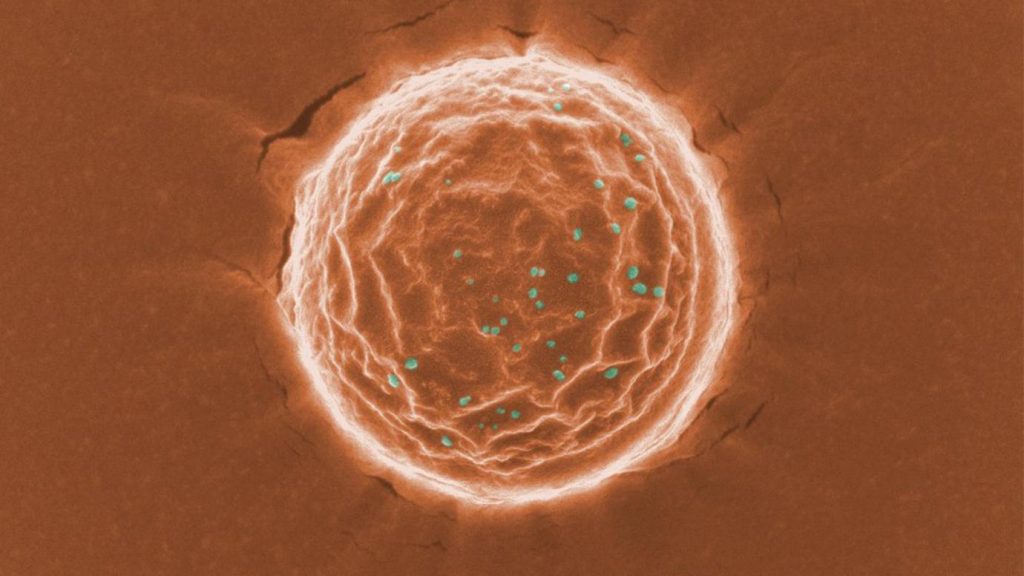
Each nanoparticle encapsulates a drug called dexamethasone sodium phosphate, one of the most commonly used corticosteroids for various ocular diseases treatment such as ocular inflammation, non-infectious uveitis, macular edema and corneal neovascularization. By using the nanoparticles to control the release of the medicine over time, patients would require only one injection right after the corneal transplantation surgery without the frequent eye drops. Our studies have shown that using this method the medication maintains its efficacy for six months on a corneal graft rejection model.
In addition, because the medicine is released slowly and directly where it is most needed, the approach requires much lower doses than current standard eyedrop treatment while providing better efficacy and safety profiles.
Qingguo Xu, D.Phil., the principal investigator of this project and an associate professor of pharmaceutics and ophthalmology at VCU School of Pharmacy, collaborated with Justin Hanes, Ph.D., the Lewis J. Ort professor of ophthalmology at Johns Hopkins University.
Xu said, “To improve patient compliance and treatment efficacy, we developed a tiny nanoparticle (around 200 nanometers) that in animal studies enables the release of the drug up to six months after a single subconjunctival injection along the eyeball.”
Tuo Meng, Ph.D., who worked on the project as a doctoral student at VCU and is the first author of this paper, said: “In our preclinical corneal graft rejection model, the single dosing of the nanoparticle successfully prevented corneal graft rejection for six months.”
More importantly, the nanoparticle approach reversed signs of early rejection and maintained corneal grafts for six months without rejection.
This work was supported by the National Eye Institute, National Institutes of Health, through the R01 grant R01EY027827.
Xu’s lab focuses on developing nanotherapeutics for safer and more effective treatment of various eye diseases.
Here’s a link to and a citation for the paper,
Six-month effective treatment of corneal graft rejection by Tuo Meng, Jinhua Zheng, Min Chen, Yang Zhao, Hadi Sudarjat, Aji Alex M.R., Vineet Kulkarni, Yumin Oh, Shiyu Xia, Zheng Ding, Hyounkoo Han, Nicole Anders, Michelle A. Rudek, Woon Chow, Walter Stark, Laura M. Ensign, Justin Hanes, and Qingguo Xu. Science Advances 22 Mar 2023 Vol 9, Issue 12 DOI: 10.1126/sciadv.adf4608
This paper is open access.