One doesn’t usually think about buckyballs (Buckminsterfullerenes) and diamondoids as being together in one molecule but that has not stopped scientists from trying to join them and, in this case, successfully. From a Sept. 9, 2014 news item on ScienceDaily,
Scientists have married two unconventional forms of carbon — one shaped like a soccer ball, the other a tiny diamond — to make a molecule that conducts electricity in only one direction. This tiny electronic component, known as a rectifier, could play a key role in shrinking chip components down to the size of molecules to enable faster, more powerful devices.
Here’s an illustration the scientists have provided,
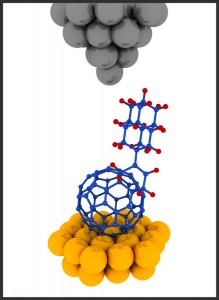
Illustration of a buckydiamondoid molecule under a scanning tunneling microscope (STM). In this study the STM made images of the buckydiamondoids and probed their electronic properties.
A Sept. 9, 2014 Stanford University news release by Glenda Chui (also on EurekAlert), which originated the news item, provides some information about this piece of international research along with background information on buckyballs and diamondoids (Note: Links have been removed),
“We wanted to see what new, emergent properties might come out when you put these two ingredients together to create a ‘buckydiamondoid,'” said Hari Manoharan of the Stanford Institute for Materials and Energy Sciences (SIMES) at the U.S. Department of Energy’s SLAC National Accelerator Laboratory. “What we got was basically a one-way valve for conducting electricity – clearly more than the sum of its parts.”
The research team, which included scientists from Stanford University, Belgium, Germany and Ukraine, reported its results Sept. 9 in Nature Communications.
Many electronic circuits have three basic components: a material that conducts electrons; rectifiers, which commonly take the form of diodes, to steer that flow in a single direction; and transistors to switch the flow on and off. Scientists combined two offbeat ingredients – buckyballs and diamondoids – to create the new diode-like component.
Buckyballs – short for buckminsterfullerenes – are hollow carbon spheres whose 1985 discovery earned three scientists a Nobel Prize in chemistry. Diamondoids are tiny linked cages of carbon joined, or bonded, as they are in diamonds, with hydrogen atoms linked to the surface, but weighing less than a billionth of a billionth of a carat. Both are subjects of a lot of research aimed at understanding their properties and finding ways to use them.
In 2007, a team led by researchers from SLAC and Stanford discovered that a single layer of diamondoids on a metal surface can emit and focus electrons into a tiny beam. Manoharan and his colleagues wondered: What would happen if they paired an electron-emitting diamondoid with another molecule that likes to grab electrons? Buckyballs are just that sort of electron-grabbing molecule.
Details are then provided about this specific piece of research (from the Stanford news release),
For this study, diamondoids were produced in the SLAC laboratory of SIMES researchers Jeremy Dahl and Robert Carlson, who are world experts in extracting the tiny diamonds from petroleum. The diamondoids were then shipped to Germany, where chemists at Justus-Liebig University figured out how to attach them to buckyballs.
The resulting buckydiamondoids, which are just a few nanometers long, were tested in SIMES laboratories at Stanford. A team led by graduate student Jason Randel and postdoctoral researcher Francis Niestemski used a scanning tunneling microscope to make images of the hybrid molecules and measure their electronic behavior. They discovered that the hybrid is an excellent rectifier: The electrical current flowing through the molecule was up to 50 times stronger in one direction, from electron-spitting diamondoid to electron-catching buckyball, than in the opposite direction. This is something neither component can do on its own.
While this is not the first molecular rectifier ever invented, it’s the first one made from just carbon and hydrogen, a simplicity researchers find appealing, said Manoharan, who is an associate professor of physics at Stanford. The next step, he said, is to see if transistors can be constructed from the same basic ingredients.
“Buckyballs are easy to make – they can be isolated from soot – and the type of diamondoid we used here, which consists of two tiny cages, can be purchased commercially,” he said. “And now that our colleagues in Germany have figured out how to bind them together, others can follow the recipe. So while our research was aimed at gaining fundamental insights about a novel hybrid molecule, it could lead to advances that help make molecular electronics a reality.”
Other research collaborators came from the Catholic University of Louvain in Belgium and Kiev Polytechnic Institute in Ukraine. The primary funding for the work came from U.S. the Department of Energy Office of Science (Basic Energy Sciences, Materials Sciences and Engineering Divisions).
Here’s a link to and a citation for the paper,
Unconventional molecule-resolved current rectification in diamondoid–fullerene hybrids by Jason C. Randel, Francis C. Niestemski, Andrés R. Botello-Mendez, Warren Mar, Georges Ndabashimiye, Sorin Melinte, Jeremy E. P. Dahl, Robert M. K. Carlson, Ekaterina D. Butova, Andrey A. Fokin, Peter R. Schreiner, Jean-Christophe Charlier & Hari C. Manoharan. Nature Communications 5, Article number: 4877 doi:10.1038/ncomms5877 Published 09 September 2014
This paper is open access. The scientists provided not only a standard illustration but a pretty picture of the buckydiamondoid,
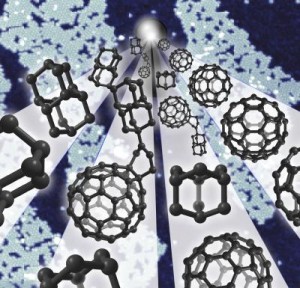
Caption: An international team led by researchers at SLAC National Accelerator Laboratory and Stanford University joined two offbeat carbon molecules — diamondoids, the square cages at left, and buckyballs, the soccer-ball shapes at right — to create “buckydiamondoids,” center. These hybrid molecules function as rectifiers, conducting electrons in only one direction, and could help pave the way to molecular electronic devices.
Credit: Manoharan Lab/Stanford University