I have two items about water. The first concerns a new technique from MIT (Massachusetts Institute of Technology) for desalination using graphene and sapwood, respectively*. From a Feb. 25, 2014 news release by David Chandler on EurekAlert,
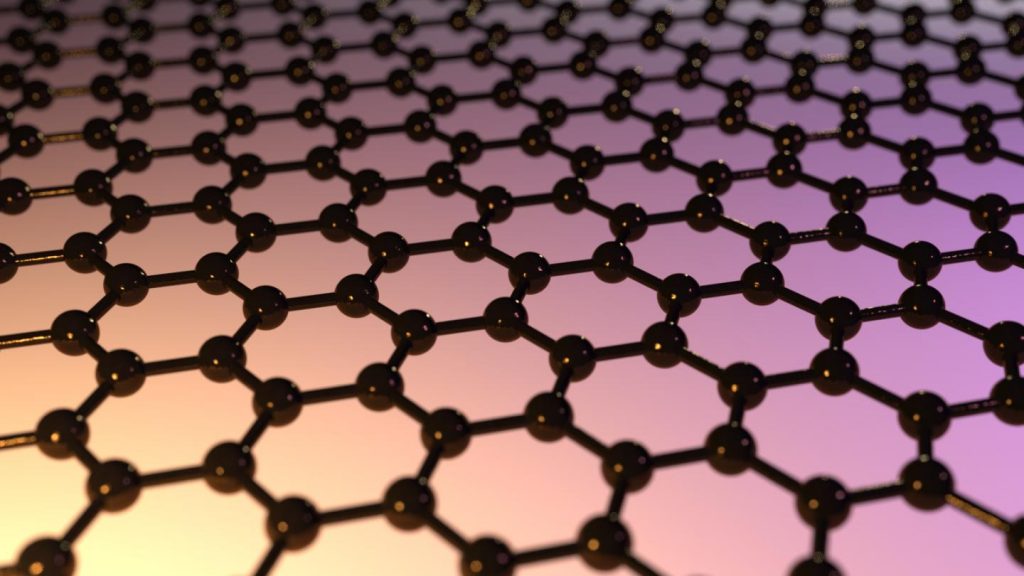
Researchers have devised a way of making tiny holes of controllable size in sheets of graphene, a development that could lead to ultrathin filters for improved desalination or water purification.
The team of researchers at MIT, Oak Ridge National Laboratory, and in Saudi Arabia succeeded in creating subnanoscale pores in a sheet of the one-atom-thick material, which is one of the strongest materials known. …
The concept of using graphene, perforated by nanoscale pores, as a filter in desalination has been proposed and analyzed by other MIT researchers. The new work, led by graduate student Sean O’Hern and associate professor of mechanical engineering Rohit Karnik, is the first step toward actual production of such a graphene filter.
Making these minuscule holes in graphene — a hexagonal array of carbon atoms, like atomic-scale chicken wire — occurs in a two-stage process. First, the graphene is bombarded with gallium ions, which disrupt the carbon bonds. Then, the graphene is etched with an oxidizing solution that reacts strongly with the disrupted bonds — producing a hole at each spot where the gallium ions struck. By controlling how long the graphene sheet is left in the oxidizing solution, the MIT researchers can control the average size of the pores.
A big limitation in existing nanofiltration and reverse-osmosis desalination plants, which use filters to separate salt from seawater, is their low permeability: Water flows very slowly through them. The graphene filters, being much thinner, yet very strong, can sustain a much higher flow. “We’ve developed the first membrane that consists of a high density of subnanometer-scale pores in an atomically thin, single sheet of graphene,” O’Hern says.
For efficient desalination, a membrane must demonstrate “a high rejection rate of salt, yet a high flow rate of water,” he adds. One way of doing that is decreasing the membrane’s thickness, but this quickly renders conventional polymer-based membranes too weak to sustain the water pressure, or too ineffective at rejecting salt, he explains.
With graphene membranes, it becomes simply a matter of controlling the size of the pores, making them “larger than water molecules, but smaller than everything else,” O’Hern says — whether salt, impurities, or particular kinds of biochemical molecules.
The permeability of such graphene filters, according to computer simulations, could be 50 times greater than that of conventional membranes, as demonstrated earlier by a team of MIT researchers led by graduate student David Cohen-Tanugi of the Department of Materials Science and Engineering. But producing such filters with controlled pore sizes has remained a challenge. The new work, O’Hern says, demonstrates a method for actually producing such material with dense concentrations of nanometer-scale holes over large areas.
“We bombard the graphene with gallium ions at high energy,” O’Hern says. “That creates defects in the graphene structure, and these defects are more chemically reactive.” When the material is bathed in a reactive oxidant solution, the oxidant “preferentially attacks the defects,” and etches away many holes of roughly similar size. O’Hern and his co-authors were able to produce a membrane with 5 trillion pores per square centimeter, well suited to use for filtration. “To better understand how small and dense these graphene pores are, if our graphene membrane were to be magnified about a million times, the pores would be less than 1 millimeter in size, spaced about 4 millimeters apart, and span over 38 square miles, an area roughly half the size of Boston,” O’Hern says.
With this technique, the researchers were able to control the filtration properties of a single, centimeter-sized sheet of graphene: Without etching, no salt flowed through the defects formed by gallium ions. With just a little etching, the membranes started allowing positive salt ions to flow through. With further etching, the membranes allowed both positive and negative salt ions to flow through, but blocked the flow of larger organic molecules. With even more etching, the pores were large enough to allow everything to go through.
Scaling up the process to produce useful sheets of the permeable graphene, while maintaining control over the pore sizes, will require further research, O’Hern says.
Karnik says that such membranes, depending on their pore size, could find various applications. Desalination and nanofiltration may be the most demanding, since the membranes required for these plants would be very large. But for other purposes, such as selective filtration of molecules — for example, removal of unreacted reagents from DNA — even the very small filters produced so far might be useful.
“For biofiltration, size or cost are not as critical,” Karnik says. “For those applications, the current scale is suitable.”
Dexter Johnson in a Feb. 26,2014 posting provides some context for and insight into the work (from the Nanoclast blog on the IEEE [Institute of Electrical and Electronics Engineers]), Note: Links have been removed,
About 18 months ago, I wrote about an MIT project in which computer models demonstrated that graphene could act as a filter in the desalination of water through the reverse osmosis (RO) method. RO is slightly less energy intensive than the predominantly used multi-stage-flash process. The hope was that the nanopores of the graphene material would make the RO method even less energy intensive than current versions by making it easier to push the water through the filter membrane.
The models were promising, but other researchers in the field said at the time it was going to be a long road to translate a computer model to a real product.
…
It would seem that the MIT researchers agreed it was worth the effort and accepted the challenge to go from computer model to a real device as they announced this week that they had developed a method for creating selective pores in graphene that make it suitable for water desalination.
Here’s a link to and a citation for the paper,
Selective Ionic Transport through Tunable Subnanometer Pores in Single-Layer Graphene Membranes by Sean C. O’Hern, Michael S. H. Boutilier, Juan-Carlos Idrobo, Yi Song, Jing Kong, Tahar Laoui, Muataz Atieh, and Rohit Karnik. Nano Lett., Article ASAP DOI: 10.1021/nl404118f Publication Date (Web): February 3, 2014
Copyright © 2014 American Chemical Society
This article is behind a paywall.
The second item is also from MIT and concerns a low-tech means of purifying water. From a Feb. 27, 2014 news item on Azonano,
If you’ve run out of drinking water during a lakeside camping trip, there’s a simple solution: Break off a branch from the nearest pine tree, peel away the bark, and slowly pour lake water through the stick. The improvised filter should trap any bacteria, producing fresh, uncontaminated water.
In fact, an MIT team has discovered that this low-tech filtration system can produce up to four liters of drinking water a day — enough to quench the thirst of a typical person.
In a paper published this week in the journal PLoS ONE, the researchers demonstrate that a small piece of sapwood can filter out more than 99 percent of the bacteria E. coli from water. They say the size of the pores in sapwood — which contains xylem tissue evolved to transport sap up the length of a tree — also allows water through while blocking most types of bacteria.
Co-author Rohit Karnik, an associate professor of mechanical engineering at MIT, says sapwood is a promising, low-cost, and efficient material for water filtration, particularly for rural communities where more advanced filtration systems are not readily accessible.
“Today’s filtration membranes have nanoscale pores that are not something you can manufacture in a garage very easily,” Karnik says. “The idea here is that we don’t need to fabricate a membrane, because it’s easily available. You can just take a piece of wood and make a filter out of it.”
The Feb. 26, 2014 news release on EurekAlert, which originated the news item, describes current filtration techniques and the advantages associated with this new low-tech approach,
There are a number of water-purification technologies on the market today, although many come with drawbacks: Systems that rely on chlorine treatment work well at large scales, but are expensive. Boiling water to remove contaminants requires a great deal of fuel to heat the water. Membrane-based filters, while able to remove microbes, are expensive, require a pump, and can become easily clogged.
Sapwood may offer a low-cost, small-scale alternative. The wood is comprised of xylem, porous tissue that conducts sap from a tree’s roots to its crown through a system of vessels and pores. Each vessel wall is pockmarked with tiny pores called pit membranes, through which sap can essentially hopscotch, flowing from one vessel to another as it feeds structures along a tree’s length. The pores also limit cavitation, a process by which air bubbles can grow and spread in xylem, eventually killing a tree. The xylem’s tiny pores can trap bubbles, preventing them from spreading in the wood.
“Plants have had to figure out how to filter out bubbles but allow easy flow of sap,” Karnik observes. “It’s the same problem with water filtration where we want to filter out microbes but maintain a high flow rate. So it’s a nice coincidence that the problems are similar.”
The news release also describes the experimental procedure the scientists followed (from the news release),
To study sapwood’s water-filtering potential, the researchers collected branches of white pine and stripped off the outer bark. They cut small sections of sapwood measuring about an inch long and half an inch wide, and mounted each in plastic tubing, sealed with epoxy and secured with clamps.
Before experimenting with contaminated water, the group used water mixed with red ink particles ranging from 70 to 500 nanometers in size. After all the liquid passed through, the researchers sliced the sapwood in half lengthwise, and observed that much of the red dye was contained within the very top layers of the wood, while the filtrate, or filtered water, was clear. This experiment showed that sapwood is naturally able to filter out particles bigger than about 70 nanometers.
However, in another experiment, the team found that sapwood was unable to separate out 20-nanometer particles from water, suggesting that there is a limit to the size of particles coniferous sapwood can filter.
…
Finally, the team flowed inactivated, E. coli-contaminated water through the wood filter. When they examined the xylem under a fluorescent microscope, they saw that bacteria had accumulated around pit membranes in the first few millimeters of the wood. Counting the bacterial cells in the filtered water, the researchers found that the sapwood was able to filter out more than 99 percent of E. coli from water.
Karnik says sapwood likely can filter most types of bacteria, the smallest of which measure about 200 nanometers. However, the filter probably cannot trap most viruses, which are much smaller in size.
The researchers have future plans (from the news release),
Karnik says his group now plans to evaluate the filtering potential of other types of sapwood. In general, flowering trees have smaller pores than coniferous trees, suggesting that they may be able to filter out even smaller particles. However, vessels in flowering trees tend to be much longer, which may be less practical for designing a compact water filter.
Designers interested in using sapwood as a filtering material will also have to find ways to keep the wood damp, or to dry it while retaining the xylem function. In other experiments with dried sapwood, Karnik found that water either did not flow through well, or flowed through cracks, but did not filter out contaminants.
“There’s huge variation between plants,” Karnik says. “There could be much better plants out there that are suitable for this process. Ideally, a filter would be a thin slice of wood you could use for a few days, then throw it away and replace at almost no cost. It’s orders of magnitude cheaper than the high-end membranes on the market today.”
Here’s a link to and a citation for the paper,
Water Filtration Using Plant Xylem by Michael S. H. Boutilier, Jongho Lee, Valerie Chambers, Varsha Venkatesh, & Rohit Karnik. PLOS One Published: February 26, 2014 DOI: 10.1371/journal.pone.0089934
This paper is open access.
One final observation, two of the researchers (Michael S. H. Boutilier & Rohit Karnik) listed as authors on the graphene/water desalination paper are also listed on the low-tech sapwood paper solution.*
* The first sentence of the this post originally stated both items were graphene-related, it has been changed to say 1… using graphene and sapwood, respectively*’ on May 8, 2015.
The last sentence of this post was changed from
‘One final observation, two of the researchers listed as authors on the graphene/water desalination paper are also listed on the low-tech sapwood paper (Michael S. H. Boutilier & Rohit Karnik).’
to this
‘One final observation, two of the researchers (Michael S. H. Boutilier & Rohit Karnik) listed as authors on the graphene/water desalination paper are also listed on the low-tech sapwood paper solution.*’ for clarity on May 8, 2015.