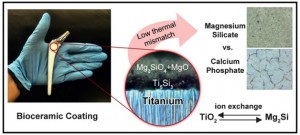
Scientists at Plymouth University (UK) have developed a nanocoating that could reduce the number of dental implant failures. From a March 24, 2017 news item on Nanowerk (Note: A link has been removed),
According to the American Academy of Implant Dentistry (AAID), 15 million Americans have crown or bridge replacements and three million have dental implants — with this latter number rising by 500,000 a year. The AAID estimates that the value of the American and European market for dental implants will rise to $4.2 billion by 2022.
Dental implants are a successful form of treatment for patients, yet according to a study published in 2005, five to 10 per cent of all dental implants fail.
The reasons for this failure are several-fold – mechanical problems, poor connection to the bones in which they are implanted, infection or rejection. When failure occurs the dental implant must be removed.
The main reason for dental implant failure is peri-implantitis. This is the destructive inflammatory process affecting the soft and hard tissues surrounding dental implants. This occurs when pathogenic microbes in the mouth and oral cavity develop into biofilms, which protects them and encourages growth. Peri-implantitis is caused when the biofilms develop on dental implants.
A research team comprising scientists from the School of Biological Sciences, Peninsula Schools of Medicine and Dentistry and the School of Engineering at the University of Plymouth, have joined forces to develop and evaluate the effectiveness of a new nanocoating for dental implants to reduce the risk of peri-implantitis.
The results of their work are published in the journal Nanotoxicology (“Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings”).
A March 27, 2017 Plymouth University press release, which originated the news item, gives more details about the research,
In the study, the research team created a new approach using a combination of silver, titanium oxide and hydroxyapatite nanocoatings.
The application of the combination to the surface of titanium alloy implants successfully inhibited bacterial growth and reduced the formation of bacterial biofilm on the surface of the implants by 97.5 per cent.
Not only did the combination result in the effective eradication of infection, it created a surface with anti-biofilm properties which supported successful integration into surrounding bone and accelerated bone healing.
Professor Christopher Tredwin, Head of Plymouth University Peninsula School of Dentistry, commented:
“In this cross-Faculty study we have identified the means to protect dental implants against the most common cause of their failure. The potential of our work for increased patient comfort and satisfaction, and reduced costs, is great and we look forward to translating our findings into clinical practice.”
The University of Plymouth was the first university in the UK to secure Research Council Funding in Nanoscience and this project is the latest in a long line of projects investigating nanotechnology and human health.
Nanoscience activity at the University of Plymouth is led by Professor Richard Handy, who has represented the UK on matters relating to the Environmental Safety and Human Health of Nanomaterials at the Organisation for Economic Cooperation and Development (OECD). He commented:
“As yet there are no nano-specific guidelines in dental or medical implant legislation and we are, with colleagues elsewhere, guiding the way in this area. The EU recognises that medical devices and implants must: perform as expected for its intended use, and be better than similar items in the market; be safe for the intended use or safer than an existing item, and; be biocompatible or have negligible toxicity.”
He added:
“Our work has been about proving these criteria which we have done in vitro. The next step would be to demonstrate the effectiveness of our discovery, perhaps with animal models and then human volunteers.”
Dr Alexandros Besinis, Lecturer in Mechanical Engineering at the School of Engineering, University of Plymouth, led the research team. He commented:
“Current strategies to render the surface of dental implants antibacterial with the aim to prevent infection and peri-implantitis development, include application of antimicrobial coatings loaded with antibiotics or chlorhexidine. However, such approaches are usually effective only in the short-term, and the use of chlorhexidine has also been reported to be toxic to human cells. The significance of our new study is that we have successfully applied a dual-layered silver-hydroxyapatite nanocoating to titanium alloy medical implants which helps to overcome these risks.”
Dr Besinis has been an Honorary Teaching Fellow at the Peninsula School of Dentistry since 2011 and has recently joined the School of Engineering. His research interests focus on advanced engineering materials and the use of nanotechnology to build novel biomaterials and medical implants with improved mechanical, physical and antibacterial properties.
Here’s a link to and a citation for the paper,
Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings by A. Besinis, S. D. Hadi, H. R. Le, C. Tredwin & R. D. Handy. Nanotoxicology Volume 11, 2017 – Issue 3 Pages 327-338 http://dx.doi.org/10.1080/17435390.2017.1299890 Published online: 17 Mar 2017
This paper appears to be open access.