Researchers at the University of Cambridge have developed a technology for producing ‘polymer opals’ on industrial scales according to a June 3, 2016 news item on Nanowerk (Note: A link has been removed),
Using a new method called Bend-Induced-Oscillatory-Shearing (BIOS), the researchers are now able to produce hundreds of metres of these materials, known as ‘polymer opals’, on a roll-to-roll process. The results are reported in the journal Nature Communications (“Large-scale ordering of nanoparticles using viscoelastic shear processing”).
A June 3, 2016 University of Cambridge press release (also on EurekAlert), which originated the news item, provides more detail (Note: Links have been removed),
Researchers have devised a new method for stacking microscopic marbles into regular layers, producing intriguing materials which scatter light into intense colours, and which change colour when twisted or stretched.
…
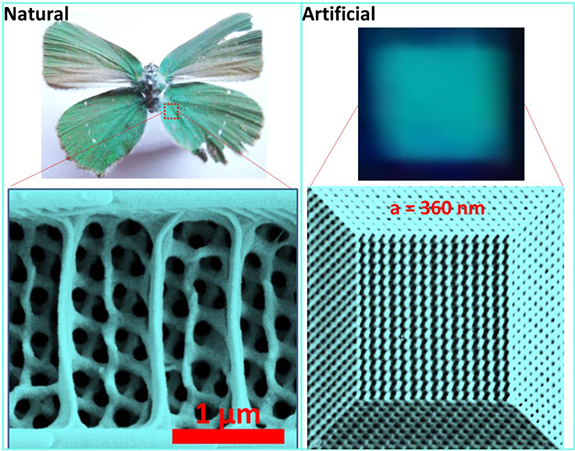
Some of the brightest colours in nature can be found in opal gemstones, butterfly wings and beetles. These materials get their colour not from dyes or pigments, but from the systematically-ordered microstructures they contain.
The team behind the current research, based at Cambridge’s Cavendish Laboratory, have been working on methods of artificially recreating this ‘structural colour’ for several years, but to date, it has been difficult to make these materials using techniques that are cheap enough to allow their widespread use.
In order to make the polymer opals, the team starts by growing vats of transparent plastic nano-spheres. Each tiny sphere is solid in the middle but sticky on the outside. The spheres are then dried out into a congealed mass. By bending sheets containing a sandwich of these spheres around successive rollers the balls are magically forced into perfectly arranged stacks, by which stage they have intense colour.
By changing the sizes of the starting nano-spheres, different colours (or wavelengths) of light are reflected. And since the material has a rubber-like consistency, when it is twisted and stretched, the spacing between the spheres changes, causing the material to change colour. When stretched, the material shifts into the blue range of the spectrum, and when compressed, the colour shifts towards red. When released, the material returns to its original colour. Such chameleon materials could find their way into colour-changing wallpapers, or building coatings that reflect away infrared thermal radiation.
I always like it when there are quotes which seem spontaneous (from the press release),
“Finding a way to coax objects a billionth of a metre across into perfect formation over kilometre scales is a miracle [emphasis mine],” said Professor Jeremy Baumberg, the paper’s senior author. “But spheres are only the first step, as it should be applicable to more complex architectures on tiny scales.”
In order to make polymer opals in large quantities, the team first needed to understand their internal structure so that it could be replicated. Using a variety of techniques, including electron microscopy, x-ray scattering, rheology and optical spectroscopy, the researchers were able to see the three-dimensional position of the spheres within the material, measure how the spheres slide past each other, and how the colours change.
“It’s wonderful [emphasis mine] to finally understand the secrets of these attractive films,” said PhD student Qibin Zhao, the paper’s lead author.
There’s also the commercialization aspect to this work (from the press release),
Cambridge Enterprise, the University’s commercialisation arm which is helping to commercialise the material, has been contacted by more than 100 companies interested in using polymer opals, and a new spin-out Phomera Technologies has been founded. Phomera will look at ways of scaling up production of polymer opals, as well as selling the material to potential buyers. Possible applications the company is considering include coatings for buildings to reflect heat, smart clothing and footwear, or for banknote security [emphasis mine] and packaging applications.
There is a Canadian company already selling its anti-counterfeiting (banknote security) bioinspired technology. It’s called Opalux and it’s not the only bioinspired anti-counterfeiting Canadian technology company, there’s also NanoTech Security which takes its inspiration from a butterfly (Blue Morpho) wing.
Getting back to Cambridge, here’s a link to and a citation for the research team’s paper,
Large-scale ordering of nanoparticles using viscoelastic shear processing by Qibin Zhao, Chris E. Finlayson, David R. E. Snoswell, Andrew Haines, Christian Schäfer, Peter Spahn, Goetz P. Hellmann, Andrei V. Petukhov, Lars Herrmann, Pierre Burdet, Paul A. Midgley, Simon Butler, Malcolm Mackley, Qixin Guo, & Jeremy J. Baumberg. Nature Communications 7, Article number: 11661 doi:10.1038/ncomms11661 Published 03 June 2016
This paper is open access.
There is a video demonstrating the stretchability of their ‘polymer opal’ film
It was posted on YouTube three years ago when the researchers were first successful. It’s nice to see they’ve been successful at getting the technology to the commercialization stage.