The Rotary Jet-Spinning manufacturing system was developed specifically as a therapeutic for the wounds of war. The dressings could be a good option for large wounds, such as burns, as well as smaller wounds on the face and hands, where preventing scarring is important. Illustration courtesy of Michael Rosnach/Harvard University
This image really gets the idea of regeneration across to the viewer while also informing you that this is medicine that comes from the military. A March 19,2018 news item on phys.org announces the work,
Researchers from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) and the Wyss Institute for Biologically Inspired Engineering have developed new wound dressings that dramatically accelerate healing and improve tissue regeneration. The two different types of nanofiber dressings, described in separate papers, use naturally-occurring proteins in plants and animals to promote healing and regrow tissue.
Our fiber manufacturing system was developed specifically for the purpose of developing therapeutics for the wounds of war,” said Kit Parker, the Tarr Family Professor of Bioengineering and Applied Physics at SEAS and senior author of the research. “As a soldier in Afghanistan, I witnessed horrible wounds and, at times, the healing process for those wounds was a horror unto itself. This research is a years-long effort by many people on my team to help with these problems.”
Parker is also a Core Faculty Member of the Wyss Institute.
The most recent paper, published in Biomaterials, describes a wound dressing inspired by fetal tissue.
A March 19, 2018 Harvard University John A. Paulson School of Engineering and Applied Science news release by Leah Burrows (also on EurekAlert), which originated the news item, provides some background information before launching into more detail about this latest work,
In the late 1970s, when scientists first started studying the wound-healing process early in development, they discovered something unexpected: Wounds incurred before the third trimester left no scars. This opened a range of possibilities for regenerative medicine. But for decades, researchers have struggled to replicate those unique properties of fetal skin.
Unlike adult skin, fetal skin has high levels of a protein called fibronectin, which assembles into the extracellular matrix and promotes cell binding and adhesion. Fibronectin has two structures: globular, which is found in blood, and fibrous, which is found in tissue. Even though fibrous fibronectin holds the most promise for wound healing, previous research focused on the globular structure, in part because manufacturing fibrous fibronectin was a major engineering challenge.
But Parker and his team are pioneers in the field of nanofiber engineering.
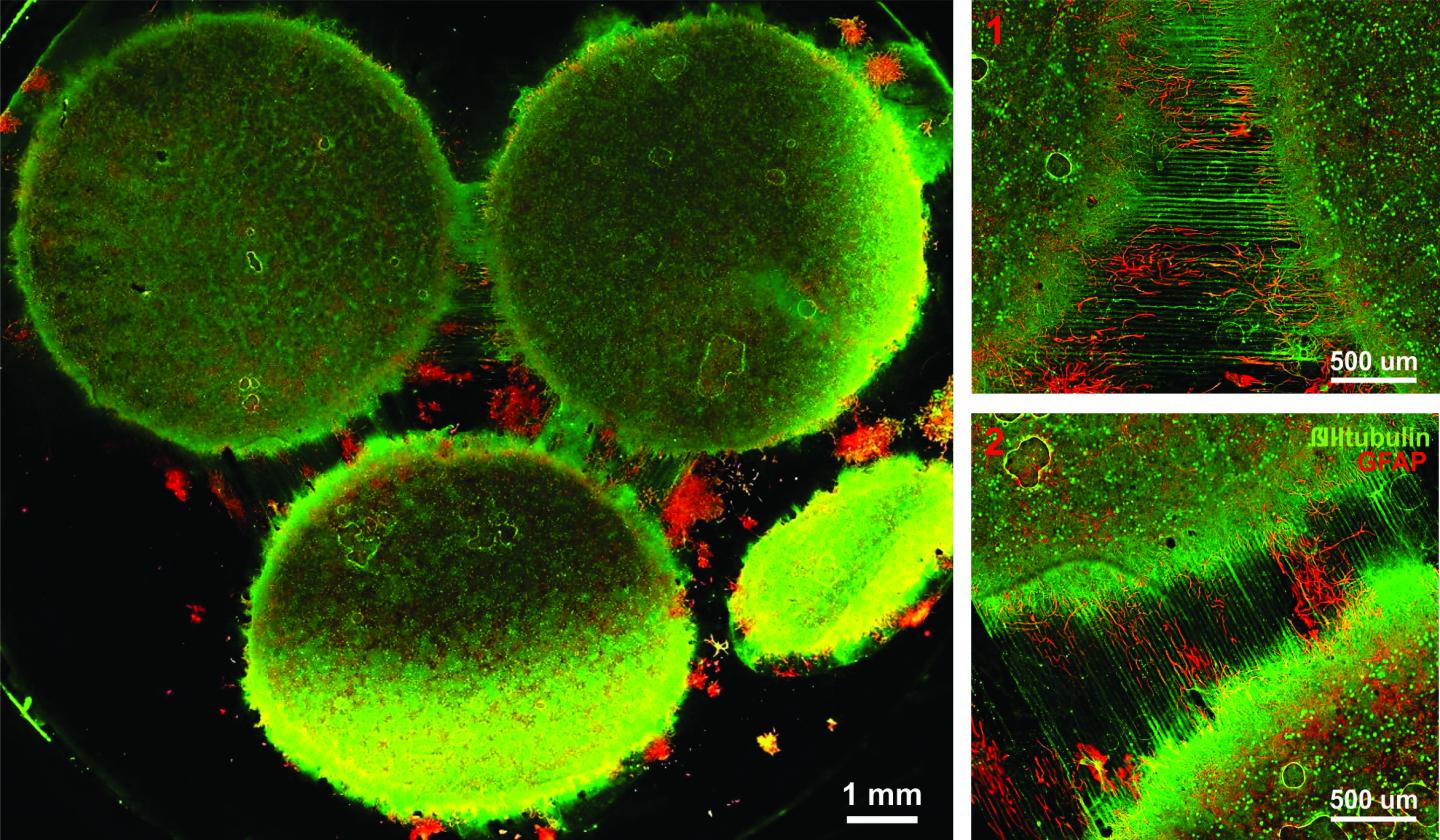
The researchers made fibrous fibronectin using a fiber-manufacturing platform called Rotary Jet-Spinning (RJS), developed by Parker’s Disease Biophysics Group. RJS works likes a cotton-candy machine — a liquid polymer solution, in this case globular fibronectin dissolved in a solvent, is loaded into a reservoir and pushed out through a tiny opening by centrifugal force as the device spins. As the solution leaves the reservoir, the solvent evaporates and the polymers solidify. The centrifugal force unfolds the globular protein into small, thin fibers. These fibers — less than one micrometer in diameter — can be collected to form a large-scale wound dressing or bandage.
“The dressing integrates into the wound and acts like an instructive scaffold, recruiting different stem cells that are relevant for regeneration and assisting in the healing process before being absorbed into the body,” said Christophe Chantre, a graduate student in the Disease Biophysics Group and first author of the paper.
In in vivo testing, the researchers found that wounds treated with the fibronectin dressing showed 84 percent tissue restoration within 20 days, compared with 55.6 percent restoration in wounds treated with a standard dressing.
The researchers also demonstrated that wounds treated with the fibronectin dressing had almost normal epidermal thickness and dermal architecture, and even regrew hair follicles — often considered one of the biggest challenges in the field of wound healing.
“This is an important step forward,” said Chantre. “Most work done on skin regeneration to date involves complex treatments combining scaffolds, cells, and even growth factors. Here we were able to demonstrate tissue repair and hair follicle regeneration using an entirely material approach. This has clear advantages for clinical translation.”
In another paper published in Advanced Healthcare Materials, the Disease Biophysics Group demonstrated a soy-based nanofiber that also enhances and promotes wound healing.
Soy protein contains both estrogen-like molecules — which have been shown to accelerate wound healing — and bioactive molecules similar to those that build and support human cells.
“Both the soy- and fibronectin-fiber technologies owe their success to keen observations in reproductive medicine,” said Parker. “During a woman’s cycle, when her estrogen levels go high, a cut will heal faster. If you do a surgery on a baby still in the womb, they have scar-less wound healing. Both of these new technologies are rooted in the most fascinating of all the topics in human biology — how we reproduce.”
In a similar way to fibronectin fibers, the research team used RJS to spin ultrathin soy fibers into wound dressings. In experiments, the soy- and cellulose-based dressing demonstrated a 72 percent increase in healing over wounds with no dressing and a 21 percent increase in healing over wounds dressed without soy protein.
“These findings show the great promise of soy-based nanofibers for wound healing,” said Seungkuk Ahn, a graduate student in the Disease Biophysics Group and first author of the paper. “These one-step, cost-effective scaffolds could be the next generation of regenerative dressings and push the envelope of nanofiber technology and the wound-care market.”
Both kinds of dressing, according to researchers, have advantages in the wound-healing space. The soy-based nanofibers — consisting of cellulose acetate and soy protein hydrolysate — are inexpensive, making them a good option for large-scale use, such as on burns. The fibronectin dressings, on the other hand, could be used for smaller wounds on the face and hands, where preventing scarring is important.
Here’s are links and citations for both papers mentioned in the news release,
Soy Protein/Cellulose Nanofiber Scaffolds Mimicking Skin Extracellular Matrix for Enhanced Wound Healing by Seungkuk Ahn, Christophe O. Chantre, Alanna R. Gannon, Johan U. Lind, Patrick H. Campbell, Thomas Grevesse, Blakely B. O’Connor, Kevin Kit Parker. Advanced Healthcare Materials https://doi.org/10.1002/adhm.201701175 First published: 23 January 2018
Production-scale fibronectin nanofibers promote wound closure and tissue repair in a dermal mouse model by Christophe O. Chantre, Patrick H. Campbell, Holly M. Golecki, Adrian T. Buganza, Andrew K. Capulli, Leila F. Deravi, Stephanie Dauth, Sean P. Sheehy, Jeffrey A.Paten. KarlGledhill, Yanne S. Doucet, Hasan E.Abaci, Seungkuk Ahn, Benjamin D.Pope, Jeffrey W.Ruberti, Simon P.Hoerstrup, Angela M.Christiano, Kevin Kit Parker. Biomaterials Volume 166, June 2018, Pages 96-108 https://doi.org/10.1016/j.biomaterials.2018.03.006 Available online 5 March 2018
Both papers are behind paywalls although you may want to check with ResearchGate where many researchers make their papers available for free.
One last comment, I noticed this at the end of Burrows’ news release,
The Harvard Office of Technology Development has protected the intellectual property relating to these projects and is exploring commercialization opportunities.
It reminded me of the patent battle between the Broad Institute (a Harvard University and Massachusetts Institute of Technology joint venture) and the University of California at Berkeley over CRISPR (clustered regularly interspaced short palindromic repeats) technology. (My March 15, 2017 posting describes the battle’s outcome.)
Lest we forget, there could be major financial rewards from this work.