Researchers from the Masssachusetts Institute of Technology (MIT) are working on a new formula for concrete based on bones, shells, and other such natural materials. From a May 25, 2016 news item on Nanowerk (Note: A link has been removed),
Researchers at MIT are seeking to redesign concrete — the most widely used human-made material in the world — by following nature’s blueprints.
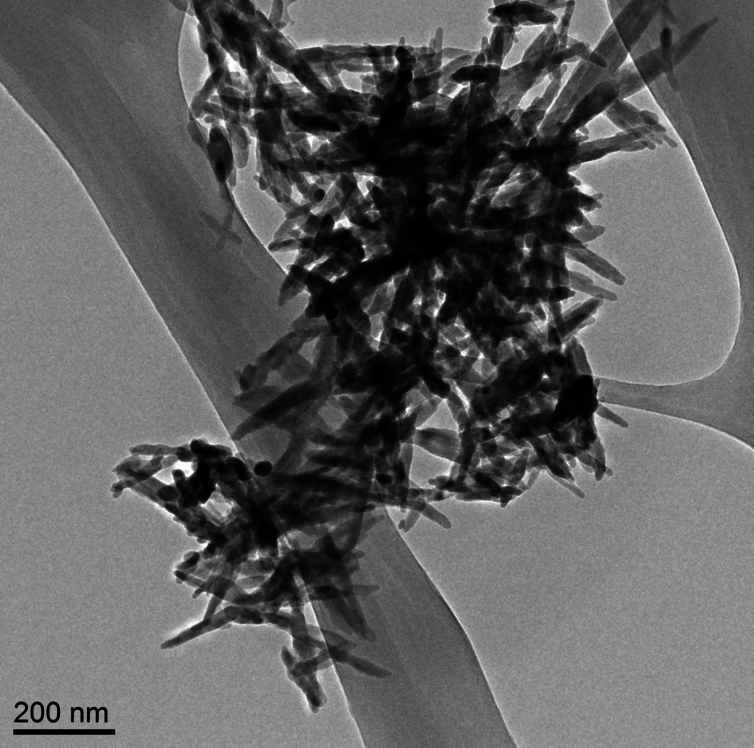
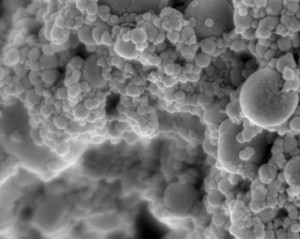
In a paper published online in the journal Construction and Building Materials (“Roadmap across the mesoscale for durable and sustainable cement paste – A bioinspired approach”), the team contrasts cement paste — concrete’s binding ingredient — with the structure and properties of natural materials such as bones, shells, and deep-sea sponges. As the researchers observed, these biological materials are exceptionally strong and durable, thanks in part to their precise assembly of structures at multiple length scales, from the molecular to the macro, or visible, level.
A May 26, 2016 MIT news release (also on EurekAlert), which originated the news item, provides more detail,
From their observations, the team, led by Oral Buyukozturk, a professor in MIT’s Department of Civil and Environmental Engineering (CEE), proposed a new bioinspired, “bottom-up” approach for designing cement paste.
“These materials are assembled in a fascinating fashion, with simple constituents arranging in complex geometric configurations that are beautiful to observe,” Buyukozturk says. “We want to see what kinds of micromechanisms exist within them that provide such superior properties, and how we can adopt a similar building-block-based approach for concrete.”
Ultimately, the team hopes to identify materials in nature that may be used as sustainable and longer-lasting alternatives to Portland cement, which requires a huge amount of energy to manufacture.
“If we can replace cement, partially or totally, with some other materials that may be readily and amply available in nature, we can meet our objectives for sustainability,” Buyukozturk says.
…
“The merger of theory, computation, new synthesis, and characterization methods have enabled a paradigm shift that will likely change the way we produce this ubiquitous material, forever,” Buehler says. “It could lead to more durable roads, bridges, structures, reduce the carbon and energy footprint, and even enable us to sequester carbon dioxide as the material is made. Implementing nanotechnology in concrete is one powerful example [of how] to scale up the power of nanoscience to solve grand engineering challenges.”
From molecules to bridges
Today’s concrete is a random assemblage of crushed rocks and stones, bound together by a cement paste. Concrete’s strength and durability depends partly on its internal structure and configuration of pores. For example, the more porous the material, the more vulnerable it is to cracking. However, there are no techniques available to precisely control concrete’s internal structure and overall properties.
“It’s mostly guesswork,” Buyukozturk says. “We want to change the culture and start controlling the material at the mesoscale.”
As Buyukozturk describes it, the “mesoscale” represents the connection between microscale structures and macroscale properties. For instance, how does cement’s microscopic arrangement affect the overall strength and durability of a tall building or a long bridge? Understanding this connection would help engineers identify features at various length scales that would improve concrete’s overall performance.
“We’re dealing with molecules on the one hand, and building a structure that’s on the order of kilometers in length on the other,” Buyukozturk says. “How do we connect the information we develop at the very small scale, to the information at the large scale? This is the riddle.”
Building from the bottom, up
To start to understand this connection, he and his colleagues looked to biological materials such as bone, deep sea sponges, and nacre (an inner shell layer of mollusks), which have all been studied extensively for their mechanical and microscopic properties. They looked through the scientific literature for information on each biomaterial, and compared their structures and behavior, at the nano-, micro-, and macroscales, with that of cement paste.
They looked for connections between a material’s structure and its mechanical properties. For instance, the researchers found that a deep sea sponge’s onion-like structure of silica layers provides a mechanism for preventing cracks. Nacre has a “brick-and-mortar” arrangement of minerals that generates a strong bond between the mineral layers, making the material extremely tough.
“In this context, there is a wide range of multiscale characterization and computational modeling techniques that are well established for studying the complexities of biological and biomimetic materials, which can be easily translated into the cement community,” says Masic.
Applying the information they learned from investigating biological materials, as well as knowledge they gathered on existing cement paste design tools, the team developed a general, bioinspired framework, or methodology, for engineers to design cement, “from the bottom up.”
The framework is essentially a set of guidelines that engineers can follow, in order to determine how certain additives or ingredients of interest will impact cement’s overall strength and durability. For instance, in a related line of research, Buyukozturk is looking into volcanic ash [emphasis mine] as a cement additive or substitute. To see whether volcanic ash would improve cement paste’s properties, engineers, following the group’s framework, would first use existing experimental techniques, such as nuclear magnetic resonance, scanning electron microscopy, and X-ray diffraction to characterize volcanic ash’s solid and pore configurations over time.
Researchers could then plug these measurements into models that simulate concrete’s long-term evolution, to identify mesoscale relationships between, say, the properties of volcanic ash and the material’s contribution to the strength and durability of an ash-containing concrete bridge. These simulations can then be validated with conventional compression and nanoindentation experiments, to test actual samples of volcanic ash-based concrete.
Ultimately, the researchers hope the framework will help engineers identify ingredients that are structured and evolve in a way, similar to biomaterials, that may improve concrete’s performance and longevity.
“Hopefully this will lead us to some sort of recipe for more sustainable concrete,” Buyukozturk says. “Typically, buildings and bridges are given a certain design life. Can we extend that design life maybe twice or three times? That’s what we aim for. Our framework puts it all on paper, in a very concrete way, for engineers to use.”
This is not the only team looking at new methods for producing the material, my Dec. 24, 2012 posting features a number of ‘concrete’ research projects.
Also, I highlighted the reference to ‘volcanic ash’ as it reminded me of Roman concrete which has lasted for over 2000 years and includes volcanic sand and volcanic rock. You can read more about it in a Dec. 18, 2014 article by Mark Miller for Ancient Origins where he describes the wonders of the material and what was then a recent discovery of the Romans’ recipe.
I have two links and citations, first, the MIT paper, then the paper on Roman concrete.
Roadmap across the mesoscale for durable and sustainable cement paste – A bioinspired approach by Steven D. Palkovic, Dieter B. Brommer, Kunal Kupwade-Patil, Admir Masic, Markus J. Buehler, Oral Büyüköztürk.Construction and Building Materials Volume 115, 15 July 2016, Pages 13–31. doi:10.1016/j.conbuildmat.2016.04.020
Mechanical resilience and cementitious processes in Imperial Roman architectural mortar by Marie D. Jackson, Eric N. Landis, Philip F. Brune, Massimo Vitti, Heng Chen, Qinfei Li, Martin Kunz, Hans-Rudolf Wenk, Paulo J. M. Monteiro, and Anthony R. Ingraffea. Proceedings of the National Academy of Sciences vol. 111 no. 52 18484–18489, doi: 10.1073/pnas.1417456111
The first paper is behind a paywall but the second one appears to be open access.