If successful the hope is that ‘human-on-a-chip’ will replace most, if not all, animal testing. This July 3, 2019 Hesperos news release (also on EurekAlert) suggests scientists are making serious gains in the drive to replace animal testing (Note: For anyone having difficulty with the terms, pharmacokinetics and pharmacodynamics, there are definitions towards the end of this posting, which may prove helpful),
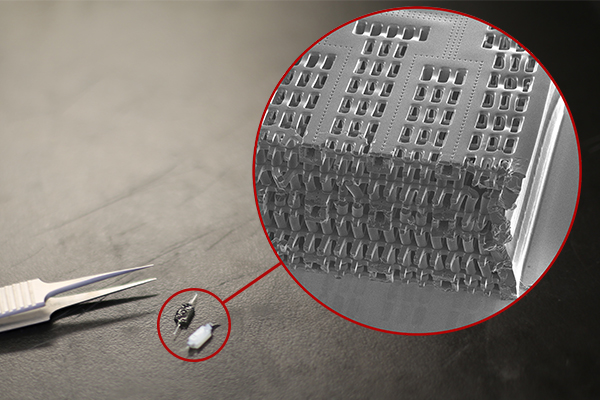
Hesperos Inc., pioneers* of the “human-on-a-chip” in vitro system has announced the use of its innovative multi-organ model to successfully measure the concentration and metabolism of two known cardiotoxic small molecules over time, to accurately describe the drug behavior and toxic effects in vivo. The findings further support the potential of body-on-a-chip systems to transform the drug discovery process.
In a study published in Nature Scientific Reports, in collaboration with AstraZeneca, Hesperos described how they used a pumpless heart model and a heart:liver system to evaluate the temporal pharmacokinetic/pharmacodynamic (PKPD) relationship for terfenadine, an antihistamine that was banned due to toxic cardiac effects, as well as determine its mechanism of toxicity.
The study found there was a time-dependent, drug-induced response in the heart model. Further experiments were conducted, adding a metabolically competent liver module to the Hesperos Human-on-a-Chip® system to observe what happened when terfenadine was converted to fexofenadine. By doing so, the researchers were able to determine the driver of the pharmacodynamic (PD) effect and develop a mathematical model to predict the effect of terfenadine in preclinical species. This is the first time an in vitro human-on-a-chip system has been shown to predict in vivo outcomes, which could be used to predict clinical trial outcomes in the future.
“The ability to examine PKPD relationships in vitro would enable us to understand compound behavior prior to in vivo testing, offering significant cost and time savings,” said Dr. Shuler, President and CEO, Hesperos, Inc and Professor Emeritus, Cornell University. “We are excited about the potential of this technology to help us ensure that potential new drug candidates have a higher probability of success during the clinical trial process.”
Understanding the inter-relationship between pharmacokinetics (PK), the drug’s time course for absorption, distribution, metabolism and excretion, and PD, the biological effect of a drug, is crucial in drug discovery and development. Scientists have learned that the maximum drug effect is not always driven by the peak drug concentration. In some cases, time is a critical factor influencing drug effect, but often this concentration-effect-time relationship only comes to light during the advanced stages of the preclinical program. In addition, often the data cannot be reliably extrapolated to humans.
“It is costly and time consuming to discover that potential drug candidates may have poor therapeutic qualities preventing their onward progression,” said James Hickman, Chief Scientist at Hesperos and Professor at the University of Central Florida. “Being able to define this during early drug discovery will be a valuable contribution to the optimization of potential new drug candidates.”
As demonstrated with the terfenadine experiment, the PKPD modelling approach was critical for understanding both the flux of compound between compartments as well as the resulting PD response in the context of dynamic exposure profiles of both parent and metabolite, as indicated by Dr. Shuler.
In order to test the viability of their system in a real-world drug discovery setting, the Hesperos team collaborated with scientists at AstraZeneca, to test one of their failed small molecules, known to have a CV [cardiovscular?] risk.
One of the main measurements used to assess the electrical properties of the heart is the QT interval, which approximates the time taken from when the cardiac ventricles start to contract to when they finish relaxing. Prolongation of the QT interval on the electrocardiogram can lead to a fatal arrhythmia known as Torsade de Pointes. Consequently, it is a mandatory requirement prior to first-in-human administration of potential new drug candidates that their ability to inhibit the hERG channel (a biomarker for QT prolongation) is investigated.
In the case of the AstraZeneca molecule, the molecule was assessed for hERG inhibition early on, and it was concluded to have a low potential to cause in vivo QT prolongation up to 100 μM. In later pre-clinical testing, the QT interval increased by 22% at a concentration of just 3 μM. Subsequent investigations found that a major metabolite was responsible. Hesperos was able to detect a clear PD effect at concentrations above 3 μM and worked to determine the mechanism of toxicity of the molecule.
The ability of these systems to assess cardiac function non-invasively in the presence of both parent molecule and metabolite over time, using multiplexed and repeat drug dosing regimes, provides an opportunity to run long-term studies for chronic administration of drugs to study their potential toxic effects.
Hesperos, Inc. is the first company spun out from the Tissue Chip Program at NCATS (National Center for Advancing Translational Sciences), which was established in 2011 to address the long timelines, steep costs and high failure rates associated with the drug development process. Hesperos currently is funded through NCATS’ Small Business Innovation Research program to undertake these studies and make tissue chips technology available as a service based company.
“The application of tissue chip technology in drug testing can lead to advances in predicting the potential effects of candidate medicines in people,” said Danilo Tagle, Ph.D., associate director for special initiatives at NCATS.
###
About Hesperos
Hesperos, Inc. is a leader in efforts to characterize an individual’s biology with human-on-a-chip microfluidic systems. Founders Michael L. Shuler and James J. Hickman have been at the forefront of every major scientific discovery in this realm, from individual organ-on-a-chip constructs to fully functional, interconnected multi-organ systems. With a mission to revolutionize toxicology testing as well as efficacy evaluation for drug discovery, the company has created pumpless platforms with serum-free cellular mediums that allow multi-organ system communication and integrated computational PKPD modeling of live physiological responses utilizing functional readouts from neurons, cardiac, muscle, barrier tissues and neuromuscular junctions as well as responses from liver, pancreas and barrier tissues. Created from human stem cells, the fully human systems are the first in vitro solutions that accurately utilize in vitro systems to predict in vivo functions without the use of animal models, as featured in Science. More information is available at http://www.
hesperosinc.com
Years ago I went to a congress focused on alternatives to animal testing (August 22, 2014 posting) and saw a video of heart cells in a petri dish (in vitro) beating in a heartlike rhythm. It was something like this,
ipscira
Published on Oct 17, 2010 https://www.youtube.com/watch?v=BqzW9Jq-OVA
I found it amazing as did the scientist who drew my attention to it. After, it’s just a collection of heart cells. How do they start beating and keep time with each other?
Getting back to the latest research, here’s a link and a citation for the paper,
On the potential of in vitro organ-chip models to define temporal pharmacokinetic-pharmacodynamic relationships by Christopher W. McAleer, Amy Pointon, Christopher J. Long, Rocky L. Brighton, Benjamin D. Wilkin, L. Richard Bridges, Narasimham Narasimhan Sriram, Kristin Fabre, Robin McDougall, Victorine P. Muse, Jerome T. Mettetal, Abhishek Srivastava, Dominic Williams, Mark T. Schnepper, Jeff L. Roles, Michael L. Shuler, James J. Hickman & Lorna Ewart. Scientific Reports volume 9, Article number: 9619 (2019) DOI: https://doi.org/10.1038/s41598-019-45656-4 Published: 03 July 2019
This paper is open access.
I happened to look at the paper and found good definitions of pharmacokinetics and pharmacodynamics. I know it’s not for everyone but if you’ve ever been curious about the difference (from the Introduction of On the potential of in vitro organ-chip models to define temporal pharmacokinetic-pharmacodynamic relationships),
Integrative pharmacology is a discipline that builds an understanding of the inter-relationship between pharmacokinetics (PK), the drug’s time course for absorption, distribution, metabolism and excretion and pharmacodynamics (PD), the biological effect of a drug. In drug discovery, this multi-variate approach guides medicinal chemists to modify structural properties of a drug molecule to improve its chance of becoming a medicine in a process known as “lead optimization”.
…
*More than one person and more than one company and more than one country claims pioneer status where ‘human-on-a-chip’ is concerned.

![Schematic of the two-organ system [downloaded from http://www.rsc.org/chemistryworld/2014/07/nanoparticle-liver-gastrointestinal-tract-microfluidic-chip]](http://www.frogheart.ca/wp-content/uploads/2014/08/TwoOrganSystem.jpg)