Finnish research is highlighted in an August 28, 2018 news item on phys.org,
A method developed at Aalto University, Finland, can produce large quantities of pristine single-walled carbon nanotubes in select shades of the rainbow. The secret is a fine-tuned fabrication process—and a small dose of carbon dioxide. The films could find applications in touch screen technologies or as coating agents for new types of solar cells.
An August 28, 2018 Aalto University press release (also on EurekAlert), which originated the news item, provides more detail,
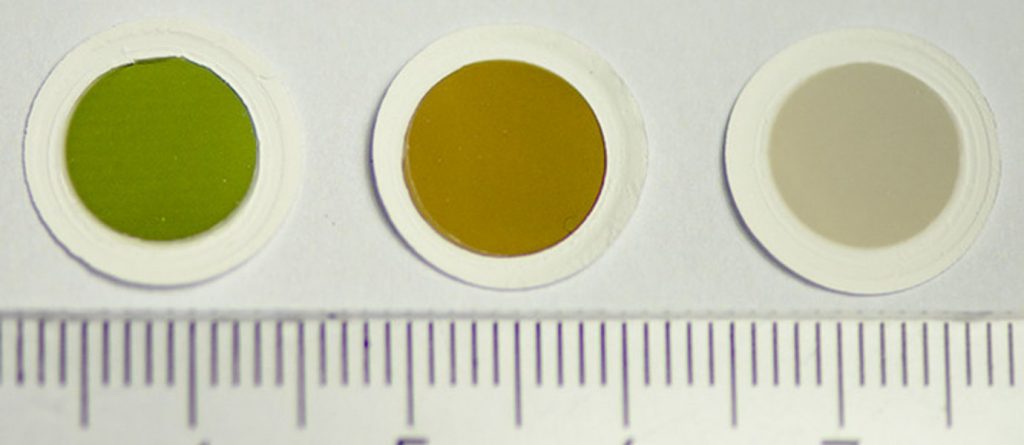
Samples of the colourful carbon nanotube thin films, as produced in the fabrication reactor. Image: Aalto University.Single-walled carbon nanotubes, or sheets of one atom-thick layers of graphene rolled up into different sizes and shapes, have found many uses in electronics and new touch screen devices. By nature, carbon nanotubes are typically black or a dark grey.
In their new study published in the Journal of the American Chemical Society (JACS), Aalto University researchers present a way to control the fabrication of carbon nanotube thin films so that they display a variety of different colours—for instance, green, brown, or a silvery grey.
The researchers believe this is the first time that coloured carbon nanotubes have been produced by direct synthesis. Using their invention, the colour is induced straight away in the fabrication process, not by employing a range of purifying techniques on finished, synthesized tubes.
With direct synthesis, large quantities of clean sample materials can be produced while also avoiding damage to the product in the purifying process—which makes it the most attractive approach for applications.
‘In theory, these coloured thin films could be used to make touch screens with many different colours, or solar cells that display completely new types of optical properties,’ says Esko Kauppinen, Professor at Aalto University.
To get carbon structures to display colours is a feat in itself. The underlying techniques needed to enable the colouration also imply finely detailed control of the structure of the nanotube structures. Kauppinen and his team’s unique method, which uses aerosols of metal and carbon, allows them to carefully manipulate and control the nanotube structure directly from the fabrication process.
‘Growing carbon nanotubes is, in a way, like planting trees: we need seeds, feeds, and solar heat. For us, aerosol nanoparticles of iron work as a catalyst or seed, carbon monoxide as the source for carbon, so feed, and a reactor gives heat at a temperature more than 850 degrees Celsius,’ says Dr. Hua Jiang, Senior Scientist at Aalto University.
Professor Kauppinen’s group has a long history of using these very resources in their singular production method. To add to their repertoire, they have recently experimented with administering small doses of carbon dioxide into the fabrication process.
‘Carbon dioxide acts as a kind of graft material that we can use to tune the growth of carbon nanotubes of various colors,’ explains Jiang.
With an advanced electron diffraction technique, the researchers were able to find out the precise atomic scale structure of their thin films. They found that they have very narrow chirality distributions, meaning that the orientation of the honeycomb-lattice of the tubes’ walls is almost uniform throughout the sample. The chirality more or less dictates the electrical properties carbon nanotubes can have, as well as their colour.
The method developed at Aalto University promises a simple and highly scalable way to fabricate carbon nanotube thin films in high yields.
‘Usually you have to choose between mass production or having good control over the structure of carbon nanotubes. With our breakthrough, we can do both,’ trusts Dr. Qiang Zhang, a postdoctoral researcher in the group.
Follow-up work is already underway.
‘We want to understand the science of how the addition of carbon dioxide tunes the structure of the nanotubes and creates colours. Our aim is to achieve full control of the growing process so that single-walled carbon nanotubes could be used as building blocks for the next generation of nanoelectronics devices,’ says professor Kauppinen.
Here’s a link to and a citation for the paper,
Direct Synthesis of Colorful Single-Walled Carbon Nanotube Thin Films by Yongping Liao, Hua Jiang, Nan Wei, Patrik Laiho, Qiang Zhang, Sabbir A. Khan, and Esko I. Kauppinen. J. Am. Chem. Soc., 2018, 140 (31), pp 9797–9800 DOI: 10.1021/jacs.8b05151 Publication Date (Web): July 26, 2018
Copyright © 2018 American Chemical Society
This paper appears to be open access.
For the curious, here’s a peek at the coloured carbon nanotube films,