Before leaping into the event details, I’ve got some information about neuromodulation for anyone who’s not familiar with the term, there are two bits (not mutually exclusive). First, there’s this Wikipedia Neuromodulation essay, which focuses on the physiological process of neuromodulation. Second, there are the answers to Frequently Asked Questions (FAQs), specifically, What is neuromodulation? on the International Neuromodulation Society (INS) website, which pertain more closely to the information being offered at the upcoming event,
…
WHAT IS NEUROMODULATION?
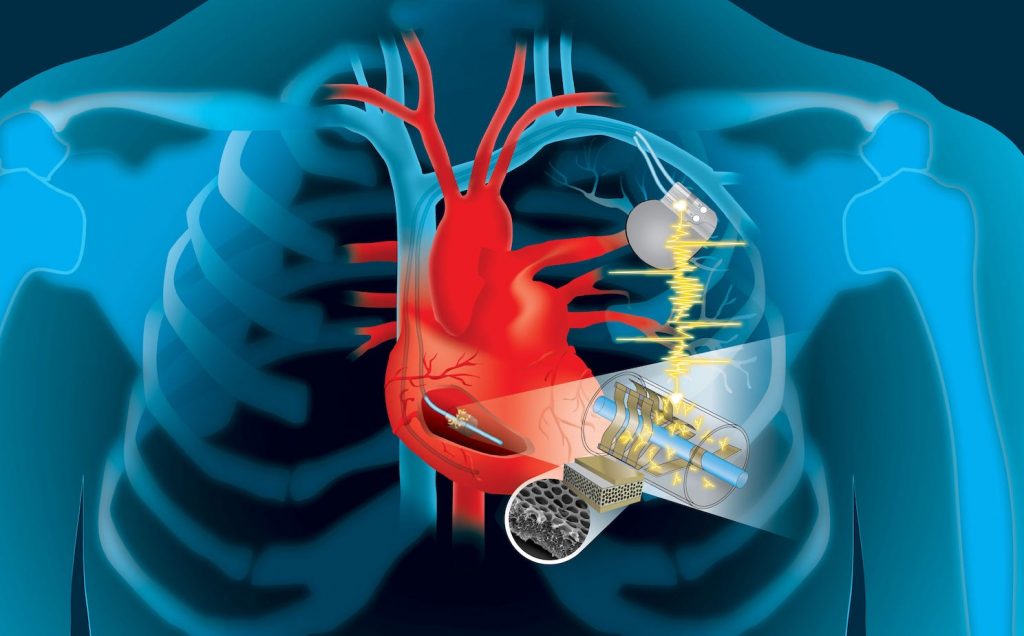
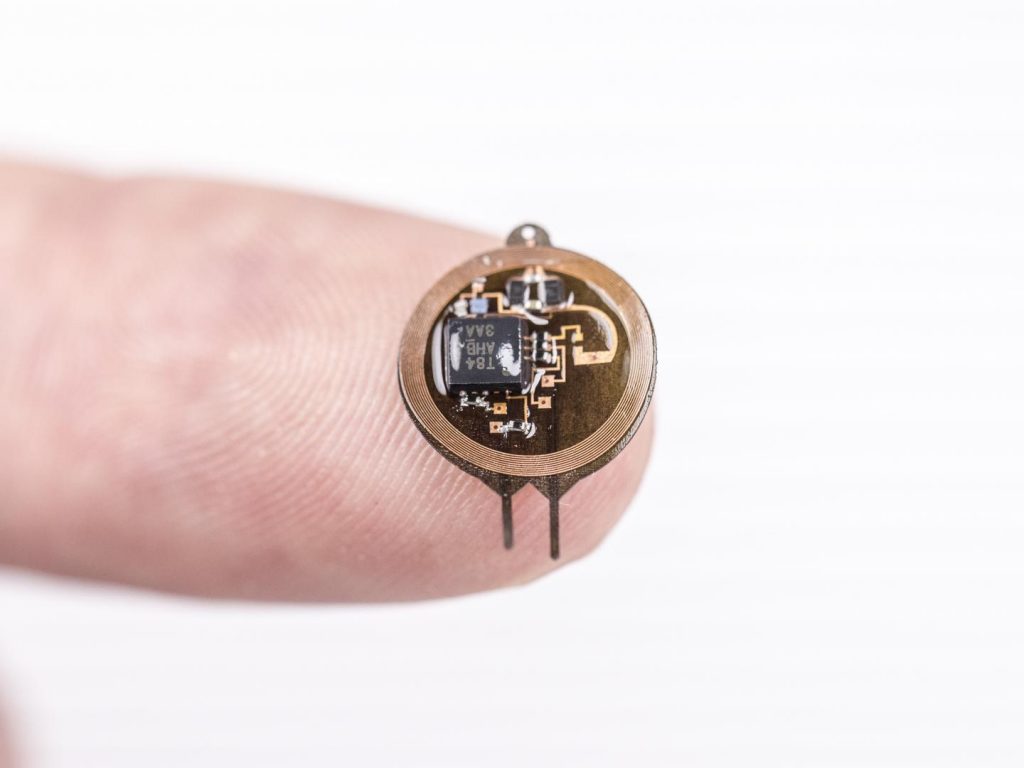
Neuromodulation is technology that acts directly upon nerves. It is the alteration—or modulation—of nerve activity by delivering electrical or pharmaceutical agents directly to a target area.
Neuromodulation devices and treatments can be life changing. They affect every area of the body and treat nearly every disease or symptom from headaches to tremors to spinal cord damage to urinary incontinence. With such a broad therapeutic scope, and significant ongoing improvements in biotechnology, it is not surprising that neuromodulation is poised as a major growth industry for the next decade.
Most frequently, people think of neuromodulation in the context of chronic pain relief, the most common indication. However, there are a plethora of neuromodulation applications, such as deep brain stimulation (DBS) treatment for Parkinson’s disease, sacral nerve stimulation for pelvic disorders and incontinence, and spinal cord stimulation for ischemic disorders (angina, peripheral vascular disease).
In addition, neuromodulation devices can stimulate a response where there was previously none, as in the case of a cochlear implant restoring hearing in a deaf patient.
And for every existing neuromodulatory treatment, there are many more on the horizon. An emerging technology called BrainGate Neural Interface System has been used to analyze brain signals and translate those signals into cursor movements, allowing severely motor-impaired individuals an alternate “pathway” to control a computer with thought, and offers potential for one day restoring some degree of limb movement.…
This April 9, 2024 International Neuromodulation Society (INS) news release on EurekAlert announces the May 11, 2024 free public event in Vancouver (Canada),
The Canadian Neuromodulation Society and the International Neuromodulation Society (INS) are delighted to announce a public education event, “Understanding Neuromodulation of the Brain and Spinal Cord”.
This complimentary event is scheduled to take place at the Vancouver Convention Centre, East Building, on Saturday, May 11, from 13:30 to 18:00, during the 16th INS World Congress.
Aimed at patients, their families, and friends dealing with conditions such as chronic pain, Parkinson’s disease, and tremor, this event is also open to interested members of the public, media representatives, and professionals.
This gathering comprises several lectures that pair scientifically and clinically substantiated insights with firsthand, real-world experiences. It provides a unique opportunity to learn directly from both local and international medical experts and patients about neuromodulation therapies. Neuromodulation treatments involve “altering nerve activity through the targeted delivery of electrical stimulation or chemical agents to specific neurological sites in the body” (Source: INS).
This event will be moderated by Dr. Christopher Honey, MD, DPhil, FRCPC, FACS, Professor & Head, Division of Neurosurgery at the University of British Columbia, as well as esteemed leader, clinician, author and INS Congress Chair.
“I am both delighted and honoured to chair this meeting. We have brought the world’s experts in neuromodulation and more than a thousand clinicians to Vancouver for the scientific meeting. The public lectures will provide background information on neuromodulation and allow our patients to give a first-hand review of their experience with the technology.”
Event Highlights:
* Educational Sessions: A series of talks covering various aspects of neuromodulation, including its application for Parkinson’s Disease, tremor, dystonia, back & leg pain, neuropathic pain (CRPS and post-surgical), and angina and peripheral vascular disease.
* Patient Experiences: Hear firsthand accounts from patients who have benefited from neuromodulation therapies, providing insights into their journeys and outcomes.
* Interactive Q&A: Dedicated Q&A sessions will allow attendees to engage with experts, ask questions, and deepen their understanding of neuromodulation and its risks and benefits.
* Networking: Opportunities for attendees to connect with healthcare professionals, researchers and others interested in neuromodulation.
This event is particularly significant as it precedes the INS 16th World Congress on Neuromodulation, highlighting the importance of public education alongside scientific discourse. It underlines the commitment of both the Canadian Neuromodulation Society and INS to raising awareness about therapies that can significantly improve the quality of life for individuals with chronic conditions.
Registration Information:
Attendance is free of charge, but registration is required. Interested participants are encouraged to register early to secure their place at this informative session.
About the International Neuromodulation Society:
The International Neuromodulation Society (INS) is a global non-profit organization focused on the scientific development and awareness of neuromodulation. The INS is dedicated to promoting improved patient care through education, research, and advocacy in the field of neuromodulation. The Canadian Neuromodulation Society has been an established chapter of the INS since 2006. [You can find the Canadian Neuromodulation Society website here.]
Good luck getting a seat!