It’s been a while since I’ve written about the Lycurgus Cup (my Sept. 21, 2010 posting). Dated from the 4th Century AD or CE, the cup is often cited as ancient nanotechnology due to certain optical properties made possible by the inclusion of nanoparticles so it glows green or red depending on the direction of the light.
A Feb. 14, 2013 news item on ScienceDaily features some work in the area of nanoplasmonics that was inspired by the cup,
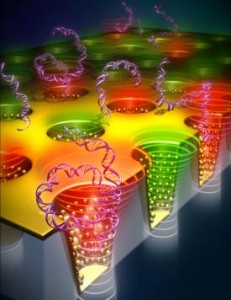
Utilizing optical characteristics first demonstrated by the ancient Romans, researchers at the University of Illinois at Urbana-Champaign have created a novel, ultra-sensitive tool for chemical, DNA, and protein analysis.
“With this device, the nanoplasmonic spectroscopy sensing, for the first time, becomes colorimetric sensing, requiring only naked eyes or ordinary visible color photography,” explained Logan Liu, an assistant professor of electrical and computer engineering and of bioengineering at Illinois. “It can be used for chemical imaging, biomolecular imaging, and integration to portable microfluidics devices for lab-on-chip-applications. His research team’s results were featured in the cover article of the inaugural edition of Advanced Optical Materials (AOM, optical section of Advanced Materials).
The Lycurgus cup was created by the Romans in 400 A.D. Made of a dichroic glass, the famous cup exhibits different colors depending on whether or not light is passing through it; red when lit from behind and green when lit from in front. It is also the origin of inspiration for all contemporary nanoplasmonics research — the study of optical phenomena in the nanoscale vicinity of metal surfaces.
The University of Illinois College of Engineering Feb. 14, 2013 news release, which originated the news item,
“This dichroic effect was achieved by including tiny proportions of minutely ground gold and silver dust in the glass,” Liu added. “In our research, we have created a large-area high density array of a nanoscale Lycurgus cup using a transparent plastic substrate to achieve colorimetric sensing. The sensor consists of about one billion nano cups in an array with sub-wavelength opening and decorated with metal nanoparticles on side walls, having similar shape and properties as the Lycurgus cups displayed in a British museum. Liu and his team were particularly excited by the extraordinary characteristics of the material, yielding 100 times better sensitivity than any other reported nanoplasmonic device.
Here’s a little more about colorimetrics and what the researchers are trying to accomplish (from the news release; Note: A link has been removed),
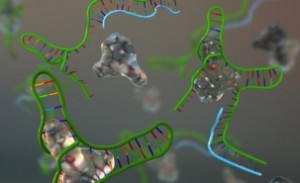
Colorimetric techniques are mainly attractive because of their low cost, use of inexpensive equipment, requirement of fewer signal transduction hardware, and above all, providing simple-to-understand results. … The current design will also enable new technology development in the field of DNA/protein microarray.
“Our label-free colorimetric sensor eliminates the need of problematic fluorescence tagging of DNA/ protein molecules, and the hybridization of probe and target molecule is detected from the color change of the sensor,” stated Manas Gartia, first author of the article, “Colorimetrics: Colorimetric Plasmon Resonance Imaging Using Nano Lycurgus Cup Arrays.” “Our current sensor requires just a light source and a camera to complete the DNA sensing process. This opens the possibility for developing affordable, simple and sensitive mobile phone-based DNA microarray detector in near future. Due to its low cost, simplicity in design, and high sensitivity, we envisage the extensive use of the device for DNA microarrays, therapeutic antibody screening for drug discovery, and pathogen detection in resource poor setting.”
…
In addition to Gartia and Liu, the paper’s co-authors included Austin Hsiao, Anusha Pokhriyal, Sujin Seo, Gulsim Kulsharova, and Brian T. Cunningham at Illinois, and Tiziana C. Bond, at the Meso, Micro and Nano Technologies Center at Lawrence Livermore National Laboratory, California.
The team’s article is behind a paywall and you can find a complete citation by clicking on the link to ScienceDaily news item.

![Among the Picasso paintings in the Art Institute of Chicago collection, The Red Armchair is the most emblematic of his Ripolin usage and is the painting that was examined with APS X-rays at Argonne National Laboratory. To view a larger version of the image, click on it. Courtesy Art Institute of Chicago, Gift of Mr. and Mrs. Daniel Saidenberg (AIC 1957.72) © Estate of Pablo Picasso / Artists Rights Society (ARS), New York [downloaded from http://www.anl.gov/articles/high-energy-x-rays-shine-light-mystery-picasso-s-paints]](http://www.frogheart.ca/wp-content/uploads/2013/02/PicassoRedArmchair.jpg)