This video is a bit technical but then it is about work being presented to chemists at the American Chemical Society’s (ACS) at the 254th National Meeting & Exposition Aug. 20 -24, 2017,
For a more plain language explanation, there’s an August 22, 2017 ACS news release (also on EurekAlert),
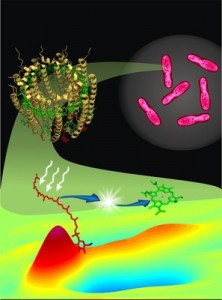
Photosynthesis provides energy for the vast majority of life on Earth. But chlorophyll, the green pigment that plants use to harvest sunlight, is relatively inefficient. To enable humans to capture more of the sun’s energy than natural photosynthesis can, scientists have taught bacteria to cover themselves in tiny, highly efficient solar panels to produce useful compounds.
…
“Rather than rely on inefficient chlorophyll to harvest sunlight, I’ve taught bacteria how to grow and cover their bodies with tiny semiconductor nanocrystals,” says Kelsey K. Sakimoto, Ph.D., who carried out the research in the lab of Peidong Yang, Ph.D. “These nanocrystals are much more efficient than chlorophyll and can be grown at a fraction of the cost of manufactured solar panels.”
Humans increasingly are looking to find alternatives to fossil fuels as sources of energy and feedstocks for chemical production. Many scientists have worked to create artificial photosynthetic systems to generate renewable energy and simple organic chemicals using sunlight. Progress has been made, but the systems are not efficient enough for commercial production of fuels and feedstocks.
Research in Yang’s lab at the University of California, Berkeley, where Sakimoto earned his Ph.D., focuses on harnessing inorganic semiconductors that can capture sunlight to organisms such as bacteria that can then use the energy to produce useful chemicals from carbon dioxide and water. “The thrust of research in my lab is to essentially ‘supercharge’ nonphotosynthetic bacteria by providing them energy in the form of electrons from inorganic semiconductors, like cadmium sulfide, that are efficient light absorbers,” Yang says. “We are now looking for more benign light absorbers than cadmium sulfide to provide bacteria with energy from light.”
Sakimoto worked with a naturally occurring, nonphotosynthetic bacterium, Moorella thermoacetica, which, as part of its normal respiration, produces acetic acid from carbon dioxide (CO2). Acetic acid is a versatile chemical that can be readily upgraded to a number of fuels, polymers, pharmaceuticals and commodity chemicals through complementary, genetically engineered bacteria.
When Sakimoto fed cadmium and the amino acid cysteine, which contains a sulfur atom, to the bacteria, they synthesized cadmium sulfide (CdS) nanoparticles, which function as solar panels on their surfaces. The hybrid organism, M. thermoacetica-CdS, produces acetic acid from CO2, water and light. “Once covered with these tiny solar panels, the bacteria can synthesize food, fuels and plastics, all using solar energy,” Sakimoto says. “These bacteria outperform natural photosynthesis.”
The bacteria operate at an efficiency of more than 80 percent, and the process is self-replicating and self-regenerating, making this a zero-waste technology. “Synthetic biology and the ability to expand the product scope of CO2 reduction will be crucial to poising this technology as a replacement, or one of many replacements, for the petrochemical industry,” Sakimoto says.
So, do the inorganic-biological hybrids have commercial potential? “I sure hope so!” he says. “Many current systems in artificial photosynthesis require solid electrodes, which is a huge cost. Our algal biofuels are much more attractive, as the whole CO2-to-chemical apparatus is self-contained and only requires a big vat out in the sun.” But he points out that the system still requires some tweaking to tune both the semiconductor and the bacteria. He also suggests that it is possible that the hybrid bacteria he created may have some naturally occurring analog. “A future direction, if this phenomenon exists in nature, would be to bioprospect for these organisms and put them to use,” he says.
For more insight into the work, check out Dexter Johnson’s Aug. 22, 2017 posting on his Nanoclast blog (on the IEEE [Institute of Electrical and Electronics Engineers] website),
“It’s actually a natural, overlooked feature of their biology,” explains Sakimoto in an e-mail interview with IEEE Spectrum. “This bacterium has a detoxification pathway, meaning if it encounters a toxic metal, like cadmium, it will try to precipitate it out, thereby detoxifying it. So when we introduce cadmium ions into the growth medium in which M. thermoacetica is hanging out, it will convert the amino acid cysteine into sulfide, which precipitates out cadmium as cadmium sulfide. The crystals then assemble and stick onto the bacterium through normal electrostatic interactions.”
I’ve just excerpted one bit, there’s more in Dexter’s posting.

![[downloaded from http://www.desy.de/infos__services/presse/pressemeldungen/@@news-view?id=9383]](http://www.frogheart.ca/wp-content/uploads/2014/11/PhotosynthesisSubsystemNearNaturalState1.jpeg)