It took me a few minutes to figure out why this item about a quadriplegic (also known as, tetraplegic) man is news. After all, I have a May 17, 2012 posting which features a video and information about a quadri(tetra)plegic woman who was drinking her first cup of coffee, independently, in many years. The difference is that she was using an external robotic arm and this man is using *his own arm*,
This Case Western Reserve University (CRWU) video accompanies a March 28, 2017 CRWU news release, (h/t ScienceDaily March 28, 2017 news item)
Bill Kochevar grabbed a mug of water, drew it to his lips and drank through the straw.
His motions were slow and deliberate, but then Kochevar hadn’t moved his right arm or hand for eight years.
And it took some practice to reach and grasp just by thinking about it.
Kochevar, who was paralyzed below his shoulders in a bicycling accident, is believed to be the first person with quadriplegia in the world to have arm and hand movements restored with the help of two temporarily implanted technologies.
A brain-computer interface with recording electrodes under his skull, and a functional electrical stimulation (FES) system* activating his arm and hand, reconnect his brain to paralyzed muscles.
Holding a makeshift handle pierced through a dry sponge, Kochevar scratched the side of his nose with the sponge. He scooped forkfuls of mashed potatoes from a bowl—perhaps his top goal—and savored each mouthful.
“For somebody who’s been injured eight years and couldn’t move, being able to move just that little bit is awesome to me,” said Kochevar, 56, of Cleveland. “It’s better than I thought it would be.”
Kochevar is the focal point of research led by Case Western Reserve University, the Cleveland Functional Electrical Stimulation (FES) Center at the Louis Stokes Cleveland VA Medical Center and University Hospitals Cleveland Medical Center (UH). A study of the work was published in the The Lancet March 28 [2017] at 6:30 p.m. U.S. Eastern time.
“He’s really breaking ground for the spinal cord injury community,” said Bob Kirsch, chair of Case Western Reserve’s Department of Biomedical Engineering, executive director of the FES Center and principal investigator (PI) and senior author of the research. “This is a major step toward restoring some independence.”
When asked, people with quadriplegia say their first priority is to scratch an itch, feed themselves or perform other simple functions with their arm and hand, instead of relying on caregivers.
“By taking the brain signals generated when Bill attempts to move, and using them to control the stimulation of his arm and hand, he was able to perform personal functions that were important to him,” said Bolu Ajiboye, assistant professor of biomedical engineering and lead study author.
Technology and training
The research with Kochevar is part of the ongoing BrainGate2* pilot clinical trial being conducted by a consortium of academic and VA institutions assessing the safety and feasibility of the implanted brain-computer interface (BCI) system in people with paralysis. Other investigational BrainGate research has shown that people with paralysis can control a cursor on a computer screen or a robotic arm (braingate.org).
“Every day, most of us take for granted that when we will to move, we can move any part of our body with precision and control in multiple directions and those with traumatic spinal cord injury or any other form of paralysis cannot,” said Benjamin Walter, associate professor of neurology at Case Western Reserve School of Medicine, clinical PI of the Cleveland BrainGate2 trial and medical director of the Deep Brain Stimulation Program at UH Cleveland Medical Center.
“The ultimate hope of any of these individuals is to restore this function,” Walter said. “By restoring the communication of the will to move from the brain directly to the body this work will hopefully begin to restore the hope of millions of paralyzed individuals that someday they will be able to move freely again.”
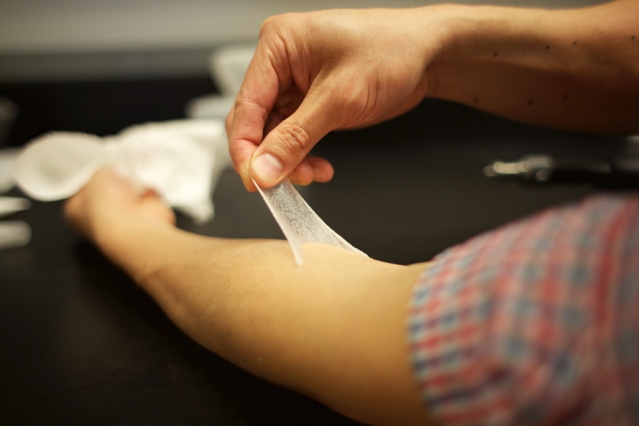
Jonathan Miller, assistant professor of neurosurgery at Case Western Reserve School of Medicine and director of the Functional and Restorative Neurosurgery Center at UH, led a team of surgeons who implanted two 96-channel electrode arrays—each about the size of a baby aspirin—in Kochevar’s motor cortex, on the surface of the brain.
The arrays record brain signals created when Kochevar imagines movement of his own arm and hand. The brain-computer interface extracts information from the brain signals about what movements he intends to make, then passes the information to command the electrical stimulation system.
To prepare him to use his arm again, Kochevar first learned how to use his brain signals to move a virtual-reality arm on a computer screen.
“He was able to do it within a few minutes,” Kirsch said. “The code was still in his brain.”
As Kochevar’s ability to move the virtual arm improved through four months of training, the researchers believed he would be capable of controlling his own arm and hand.
Miller then led a team that implanted the FES systems’ 36 electrodes that animate muscles in the upper and lower arm.
The BCI decodes the recorded brain signals into the intended movement command, which is then converted by the FES system into patterns of electrical pulses.
The pulses sent through the FES electrodes trigger the muscles controlling Kochevar’s hand, wrist, arm, elbow and shoulder. To overcome gravity that would otherwise prevent him from raising his arm and reaching, Kochevar uses a mobile arm support, which is also under his brain’s control.
New Capabilities
Eight years of muscle atrophy required rehabilitation. The researchers exercised Kochevar’s arm and hand with cyclical electrical stimulation patterns. Over 45 weeks, his strength, range of motion and endurance improved. As he practiced movements, the researchers adjusted stimulation patterns to further his abilities.
Kochevar can make each joint in his right arm move individually. Or, just by thinking about a task such as feeding himself or getting a drink, the muscles are activated in a coordinated fashion.
When asked to describe how he commanded the arm movements, Kochevar told investigators, “I’m making it move without having to really concentrate hard at it…I just think ‘out’…and it goes.”
Kocehvar is fitted with temporarily implanted FES technology that has a track record of reliable use in people. The BCI and FES system together represent early feasibility that gives the research team insights into the potential future benefit of the combined system.
Advances needed to make the combined technology usable outside of a lab are not far from reality, the researchers say. Work is underway to make the brain implant wireless, and the investigators are improving decoding and stimulation patterns needed to make movements more precise. Fully implantable FES systems have already been developed and are also being tested in separate clinical research.
Kochevar welcomes new technology—even if it requires more surgery—that will enable him to move better. “This won’t replace caregivers,” he said. “But, in the long term, people will be able, in a limited way, to do more for themselves.”
There is more about the research in a March 29, 2017 article by Sarah Boseley for The Guardian,
Bill Kochevar, 53, has had electrical implants in the motor cortex of his brain and sensors inserted in his forearm, which allow the muscles of his arm and hand to be stimulated in response to signals from his brain, decoded by computer. After eight years, he is able to drink and feed himself without assistance.
“I think about what I want to do and the system does it for me,” Kochevar told the Guardian. “It’s not a lot of thinking about it. When I want to do something, my brain does what it does.”
The experimental technology, pioneered by the Case Western Reserve University in Cleveland, Ohio, is the first in the world to restore brain-controlled reaching and grasping in a person with complete paralysis.
For now, the process is relatively slow, but the scientists behind the breakthrough say this is proof of concept and that they hope to streamline the technology until it becomes a routine treatment for people with paralysis. In the future, they say, it will also be wireless and the electrical arrays and sensors will all be implanted under the skin and invisible.
A March 28, 2017 Lancet news release on EurekAlert provides a little more technical insight into the research and Kochevar’s efforts,
Although only tested with one participant, the study is a major advance and the first to restore brain-controlled reaching and grasping in a person with complete paralysis. The technology, which is only for experimental use in the USA, circumvents rather than repairs spinal injuries, meaning the participant relies on the device being implanted and switched on to move.
“Our research is at an early stage, but we believe that this neuro-prosthesis could offer individuals with paralysis the possibility of regaining arm and hand functions to perform day-to-day activities, offering them greater independence,” said lead author Dr Bolu Ajiboye, Case Western Reserve University, USA. “So far it has helped a man with tetraplegia to reach and grasp, meaning he could feed himself and drink. With further development, we believe the technology could give more accurate control, allowing a wider range of actions, which could begin to transform the lives of people living with paralysis.” [1]
…
Previous research has used similar elements of the neuro-prosthesis. For example, a brain-computer interface linked to electrodes on the skin has helped a person with less severe paralysis open and close his hand, while other studies have allowed participants to control a robotic arm using their brain signals. However, this is the first to restore reaching and grasping via the system in a person with a chronic spinal cord injury.
In this study, a 53 year-old man who had been paralysed below the shoulders for eight years underwent surgery to have the neuro-prosthesis fitted.
This involved brain surgery to place sensors in the motor cortex area of his brain responsible for hand movement – creating a brain-computer interface that learnt which movements his brain signals were instructing for. This initial stage took four months and included training using a virtual reality arm.
He then underwent another procedure placing 36 muscle stimulating electrodes into his upper and lower arm, including four that helped restore finger and thumb, wrist, elbow and shoulder movements. These were switched on 17 days after the procedure, and began stimulating the muscles for eight hours a week over 18 weeks to improve strength, movement and reduce muscle fatigue.
The researchers then wired the brain-computer interface to the electrical stimulators in his arm, using a decoder (mathematical algorithm) to translate his brain signals into commands for the electrodes in his arm. The electrodes stimulated the muscles to produce contractions, helping the participant intuitively complete the movements he was thinking of. The system also involved an arm support to stop gravity simply pulling his arm down.
During his training, the participant described how he controlled the neuro-prosthesis: “It’s probably a good thing that I’m making it move without having to really concentrate hard at it. I just think ‘out’ and it just goes.”
After 12 months of having the neuro-prosthesis fitted, the participant was asked to complete day-to-day tasks, including drinking a cup of coffee and feeding himself. First of all, he observed while his arm completed the action under computer control. During this, he thought about making the same movement so that the system could recognise the corresponding brain signals. The two systems were then linked and he was able to use it to drink a coffee and feed himself.
He successfully drank in 11 out of 12 attempts, and it took him roughly 20-40 seconds to complete the task. When feeding himself, he did so multiple times – scooping forkfuls of food and navigating his hand to his mouth to take several bites.
“Although similar systems have been used before, none of them have been as easy to adopt for day-to-day use and they have not been able to restore both reaching and grasping actions,” said Dr Ajiboye. “Our system builds on muscle stimulating electrode technology that is already available and will continue to improve with the development of new fully implanted and wireless brain-computer interface systems. This could lead to enhanced performance of the neuro-prosthesis with better speed, precision and control.” [1]
At the time of the study, the participant had had the neuro-prosthesis implanted for almost two years (717 days) and in this time experienced four minor, non-serious adverse events which were treated and resolved.
Despite its achievements, the neuro-prosthesis still had some limitations, including that movements made using it were slower and less accurate than those made using the virtual reality arm the participant used for training. When using the technology, the participant also needed to watch his arm as he lost his sense of proprioception – the ability to intuitively sense the position and movement of limbs – as a result of the paralysis.
Writing in a linked Comment, Dr Steve Perlmutter, University of Washington, USA, said: “The goal is futuristic: a paralysed individual thinks about moving her arm as if her brain and muscles were not disconnected, and implanted technology seamlessly executes the desired movement… This study is groundbreaking as the first report of a person executing functional, multi-joint movements of a paralysed limb with a motor neuro-prosthesis. However, this treatment is not nearly ready for use outside the lab. The movements were rough and slow and required continuous visual feedback, as is the case for most available brain-machine interfaces, and had restricted range due to the use of a motorised device to assist shoulder movements… Thus, the study is a proof-of-principle demonstration of what is possible, rather than a fundamental advance in neuro-prosthetic concepts or technology. But it is an exciting demonstration nonetheless, and the future of motor neuro-prosthetics to overcome paralysis is brighter.”
…
[1] Quote direct from author and cannot be found in the text of the Article.
Here’s a link to and a citation for the paper,
Restoration of reaching and grasping movements through brain-controlled muscle stimulation in a person with tetraplegia: a proof-of-concept demonstration by A Bolu Ajiboye, Francis R Willett, Daniel R Young, William D Memberg, Brian A Murphy, Jonathan P Miller, Benjamin L Walter, Jennifer A Sweet, Harry A Hoyen, Michael W Keith, Prof P Hunter Peckham, John D Simeral, Prof John P Donoghue, Prof Leigh R Hochberg, Prof Robert F Kirsch. The Lancet DOI: http://dx.doi.org/10.1016/S0140-6736(17)30601-3 Published: 28 March 2017 [online?]
This paper is behind a paywall.
For anyone who’s interested, you can find the BrainGate website here.
*I initially misidentified the nature of the achievement and stated that Kochevar used a “robotic arm, which is attached to his body” when it was his own reanimated arm. Corrected on April 25, 2017.