I got more than I expected from both books (“The Science of Orphan Black: The Official Companion” by Casey Griffin and Nina Nesseth and “Star Trek Treknology; The Science of Star Trek from Tricorders to Warp Drive” by Ethan Siegel) I’m going to discuss by changing my expectations.
The Science of Orphan Black: The Official Companion
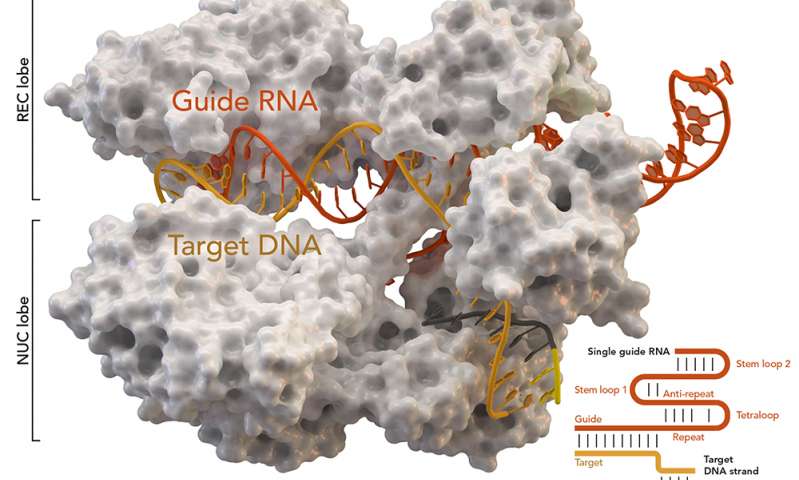
I had expected a book about the making of the series with a few insider stories about the production along with some science. Instead, I was treated to a season by season breakdown of the major scientific and related ethical issues in the fields of cloning and genetics.I don’t follow those areas exhaustively but from my inexpert perspective, the authors covered everything I could have hoped for (e.g., CRISPR/CAS9, Henrietta Lacks, etc.) in an accessible but demanding writing style In other words, it’s a good read but it’s not a light read.
There are many, many pictures of Tatiana Maslany as one of her various clone identities in the book. Unfortunately, the images do not boast good reproduction values. This was disconcerting as it can lead a reader (yes, that was me) to false expectations (e.g., this is a picture book) concerning the contents. The boxed snippets from the scripts and explanatory notes inset into the text helped to break up some of the more heavy going material while providing additional historical/scripting/etc. perspectives. One small niggle, the script snippets weren’t always as relevant to the discussion at hand as the authors no doubt hoped.
I suggest reading both the Foreword by Cosima Herter, the series science consultant, and (although it could have done with a little editing) The Conversation between Cosima Herter and Graeme Manson (one of the producers). That’s where you’ll find that the series seems to have been incubated in Vancouver, Canada. It’s also where you’ll find out how much of Cosima Herter’s real life story is included in the Cosima clone’s life story.
The Introduction tells you how the authors met (as members of ‘the clone club’) and started working together as recappers for the series. (For anyone unfamiliar with the phenomenon or terminology, episodes of popular series are recapitulated [recapped] on one or more popular websites. These may or may not be commercial, i.e., some are fan sites.)
One of the authors, Casey Griffin, is a PhD candidate at the University of Southern California (USC) studying in the field of developmental and stem cell biology. I was not able to get much more information but did find her LinkedIn profile. The other author also has a science background. Nina Nesseth is described as a science communicator on the back cover of the book but she’s described as a staff scientist for Science North, a science centre located in Sudbury, Ontario, Canada. Her LinkedIn profile lists an honours Bachelor of Science (Biological and Medical Sciences) from Laurentian University, also located in Sudbury, Ontario.
It’s no surprise, given the authors’ educational background, that a bibliography (selected) has been included. This is something I very much appreciated. Oddly, given that Nesseth lists a graduate certificate in publishing as one of her credentials (on LinkedIn), there is no index (!?!). Unusually, the copyright page is at the back of the book instead of the front and boasts a fairly harsh copyright notice (summary: don’t copy anything, ever … unless you get written permission from ECW Press and the other copyright owners; Note: Herter is the copyright owner of her Foreword while the authors own the rest).
There are logos on the copyright page—more than I’m accustomed to seeing. Interestingly, two of them are government logos. It seems that taxpayers contributed to the publication of this book. The copyright notice seems a little facey to me since taxpayers (at least partially) subsidized the book, as well, Canadian copyright law has a concept called fair dealing (in the US, there’s something similar: fair use). In other words, if I chose, I could copy portions of the text without asking for permission if there’s no intent to profit from it and as long as I give attributions.
How, for example, could anyone profit from this?
In fact, in January 2017, Jun Wu and colleagues published their success in creating pig-human hybrids. (description of real research on chimeras on p. 98)
Or this snippet of dialogue,
[Charlotte] You’re my big sister.
[Sarah] How old are you? (p. 101)
All the quoted text is from “The Science of Orphan Black: The Official Companion” by Casey Griffin and Nina Nesseth (paperback published August 22, 2017).
On the subject of chimeras, the Canadian Broadcasting Corporation (CBC) featured a January 26, 2017 article about the pig-human chimeras on its website along with a video,
Getting back to the book, copyright silliness aside, it’s a good book for anyone interested in some of the science and the issues associated with biotechnology, synthetic biology, genomes, gene editing technologies, chimeras, and more. I don’t think you need to have seen the series in order to appreciate the book.
Star Trek Treknology; The Science of Star Trek from Tricorders to Warp Drive
This looks and feels like a coffee table book. The images in this book are of a much higher quality than those in the ‘Orphan Black’ book. With thicker paper and extensive ink coverage lending to its glossy, attractive looks, it’s a physically heavy book. The unusually heavy use of black ink would seem to be in service of conveying the feeling that you are exploring the far reaches of outer space.
It’s clear that “Star Trek Treknology; The Science of Star Trek from Tricorders to Warp Drive’s” author, Ethan Siegel, PhD., is a serious Star Trek and space travel fan. All of the series and movies are referenced at one time or another in the book in relationship to technology (treknology).
Unlike Siegel, while I love science fiction and Star Trek, I have never been personally interested in space travel. Regardless, Siegel did draw me in with his impressive ability to describe and explain physics-related ideas. Unfortunately, his final chapter on medical and biological ‘treknology’ is not as good. He covers a wide range of topics but no one is an expert on everything.
Siegel has a Wikipedia entry, which notes this (Note: Links have been removed),
Ethan R. Siegel (August 3, 1978, Bronx)[1] is an American theoretical astrophysicist and science writer, who studies Big Bang theory. He is a professor at Lewis & Clark College and he blogs at Starts With a Bang, on ScienceBlogs and also on Forbes.com since 2016.
By contrast with the ‘Orphan Black’ book, the tone is upbeat. It’s one of the reasons Siegel appreciates Star Trek in its various iterations,
As we look at the real-life science and technology behind the greatest advances anticipated by Star Trek, it’s worth remembering that the greatest legacy of the show is its message of hope. The future can be brighter and better than our past or present has ever been. It’s our continuing mission to make it so. (p. 6)
All the quoted text is from “Star Trek Treknology; The Science of Star Trek from Tricorders to Warp Drive” by Ethan Siegel (hard cover published October 15, 2017).
This book too has one of those copyright notices that fail to note you don’t need permission when it’s fair dealing to copy part of the text. While it does have an index, it’s on the anemic side and, damningly, there are neither bibliography nor reference notes of any sort. If Siegel hadn’t done such a good writing job, I might not have been so distressed.
For example, it’s frustrating for someone like me who’s been trying to get information on cortical/neural implants and finds this heretofore unknown and intriguing tidbit in Siegel’s text,
In 2016, the very first successful cortical implant into a patient with ALS [amyotrophic lateral sclerosis] was completed, marking the very first fully implanted brain-computer interface in a human being. (p. 180)
Are we talking about the Australia team, which announced human clinical trials for their neural/cortical implant (my February 15, 2016 posting) or was it preliminary work by a team in Ohio (US) which later (?) announced a successful implant for a quadriplegic (also known as tetraplegic) patient who was then able to move hands and fingers (see my April 19, 2016 posting)? Or is it an entirely different team?
One other thing, I was a bit surprised to see no mention of quantum or neuromorphic computing in the chapter on computing. I don’t believe either was part of the Star Trek universe but they (neuromorphic and quantum computing) are important developments and Siegel makes a point, on at least a few occasions, of contrasting present day research with what was and wasn’t ‘predicted’ by Star Trek.
As for the ‘predictions’, there’s a longstanding interplay between storytellers and science and sometimes it can be a little hard to figure out which came first. I think Siegel might have emphasized that give and take a bit more.
Regardless of my nitpicking, Siegel is a good writer and managed to put an astonishing amount of ‘educational’ material into a lively and engaging book. That is not easy.
Final thoughts
I enjoyed both books and am very excited to see grounded science being presented along with the fictional stories of both universes (Star Trek and Orphan Black).
Yes, both books have their shortcomings (harsh copyright notices, no index, no bibliography, no reference notes, etc.) but in the main they offer adults who are sufficiently motivated a wealth of current scientific and technical information along with some elucidation of ethical issues.



![AquaBounty's salmon (background) has been genetically modified to grow bigger and faster than a conventional Atlantic salmon of the same age (foreground.) Courtesy of AquaBounty Technologies, Inc. [downloaded from http://www.npr.org/sections/thesalt/2015/06/24/413755699/genetically-modified-salmon-coming-to-a-river-near-you]](http://www.frogheart.ca/wp-content/uploads/2015/12/GeneticallyModifiedSalmon.jpg)